Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4541
Peer-review started: August 30, 2021
First decision: November 17, 2021
Revised: December 3, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
The clinicopathological features, immunohistochemical characteristics, and genetic mutation profile of two unusual cases of distal bronchiolar adenoma are retrospectively analyzed and the relevant literature is reviewed.
Case 1 was a 63-year-old female patient who had a mixed ground-glass nodule, with mild cells in morphology, visible cilia, and bilayer structures in focal areas. Immunohistochemical staining for P63 and cytokeratin (CK)5/6 revealed the lack of a continuous bilayer structure in most areas, and no mutations were found in epidermal growth factor receptor, anaplastic lymphoma kinase, ROS1, Kirsten rat sarcoma, PIK3CA, BRAF, human epidermal growth factor receptor-2 (HER2), RET, and neuroblastoma RAS genes. Case 2 was a 58-year-old female patient who presented with a solid nodule, in which most cells were observed to be medium sized, the nuclear chromatin was pale and homogeneous, local cells had atypia, and cilia were found locally. Immunohistochemical staining for P63 and CK5/6 showed no expression of these proteins in mild cell morphology whereas the heteromorphic cells showed a bilayer structure. The same nine genes as above were analyzed, and HER2 gene mutation was identified.
Some unresolved questions remain to be answered to determine whether the lesion is a benign adenoma or a part of the process of malignant transformation from benign adenoma of the bronchial epithelium. Furthermore, whether lesions with atypical bilayer structures are similar to atypical hyperplastic lesions of the breast remains to be elucidated. Moreover, clarity on whether these lesions can be called atypical bronchiolar adenoma and whether they are invasive precursor lesions is needed. Future studies should examine the diagnostic significance of HER2 gene mutation as a prognostic indicator.
Core Tip: In terms of morphology, case 1 had no atypical cells, visible cilia, and bilayer structures in focal areas. Immunohistochemical staining for P63 and cytokeratin (CK)5/6 revealed the lack of a continuous bilayer structure in most areas, and no mutations were found in the genes detected. In case 2, most cells were medium-sized. Furthermore, local cells had atypia, and cilia were found locally. Immunohistochemical staining for P63 and CK5/6 revealed that only heteromorphic cell regions showed a bilayer structure. Human epidermal growth factor receptor-2 gene mutation was identified. Further research is needed to investigate whether these lesions can be called atypical bronchiolar adenoma and whether they are invasive precursor lesions.
- Citation: Du Y, Wang ZY, Zheng Z, Li YX, Wang XY, Du R. Bronchiolar adenoma with unusual presentation: Two case reports. World J Clin Cases 2022; 10(14): 4541-4549
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4541.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4541
Bronchiolar adenoma (BA) clinically presents as a benign or potentially malignant tumor. It is thought to originate from the bronchiolar epithelium, which has a series of cell differentiation in a bilayer arrangement of multipartite epithelial cells and basal cells. BA is expected to gain more widespread recognition in the 2021 edition of the World Health Organization classification of thoracic tumors[1,2]. The histological variants of BA can be distinguished as the “classic” ciliated muconodular papillary tumor (CMPT) (proximal type) and “non-classic” CMPT (distal type). The histological features of CMPT include a bilayer structure composed of a continuous basal cell layer and luminal cell layer (comprising varying proportions of mucinous cells, ciliated cells, Clara cells, and/or type II alveolar epithelial cells)[3,4]. BA often exhibits only focal or no papillary architecture and contains variable numbers of ciliated and mucinous cells, with some lesions entirely lacking one or both of these components[3,4]. A recent study revealed the involvement of potential gene mutations that may be responsible for the neoplastic nature of BA[5]. Mutations in the anaplastic lymphoma kinase (ALK), Kirsten rat sarcoma (KRAS), BRAF, AKT1, and epidermal growth factor receptor (EGFR) genes were identified in BA, and these genes were considered as driver oncogenes that eventually lead to the development of neoplasms[6-9]. Meanwhile, in a recent study on BA, Chang et al[2] identified BRAF V600E mutations (38%), EGFR exon 19 deletions (10%), EGFR exon 20 insertions (10%), KRAS mutations (24%), and HRAS mutations (5%), thus supporting a truly neoplastic process of BA.
Cases of single- or double-layer bronchial adenoma with atypical bronchiolitis are rare. Here, we report two cases with BA confirmed by imaging, morphology examination, immunohistochemical characteristics, and genetic tests.
Case 1: A 63-year-old female patient was found to have pulmonary nodules on examination at a local hospital in September 2020.
Case 2: A 58-year-old female patient underwent chest computed tomography (CT) examination at our hospital on January 19, 2021 and was identified as having nodules in the right upper lobe of the lung.
Case 1: Upon examination at a local hospital in September 2020, the patient was found to have pulmonary nodules; she did not report having cough or expectoration, chest pain, chest tightness, or other symptoms. No further specific diagnosis was made or treatment advised. Since the discovery of the nodules, the patient has been lucid and mentally healthy with normal diet and sleep. The laboratory reports for urine and stool were normal, and there were no significant changes in weight.
Case 2: The patient underwent chest CT examination at our hospital on January 19, 2021 and was identified as having nodules in the right upper lobe of the lung. Except for occasional cough and phlegm, she showed no other signs or symptoms.
The patients had a free previous medical history.
The patients had no personal and family history.
Case 1: After admission to the hospital, the patient’s temperature was 36.6 ℃, heart rate was 58 bpm, respiratory rate was 16 breaths per minute, and blood pressure was 112/59 mmHg.
Case 2: The patient’s temperature was 36.9 ℃, heart rate was 67 bpm, respiratory rate was 16 breaths per minute, and blood pressure was 120/67 mmHg.
In both cases, chest examination found that the trachea was in the center, the thorax was not deformed, the breath sounds of the lungs were slightly thicker, and no obvious dry or wet rales were heard.
Case 1: The biochemical indicators showed the following results: Carcinoembryonic antigen (CEA) was 0.71 ng/mL (reference range: 0-5 ng/mL), neuron-specific enolase (NSE) was 11.94 ng/mL (reference range: 0-35 ng/mL), cytokeratin protein (CK19) was 2.33 ng/mL (reference range: 0-3.3 ng/mL), squamous cell carcinoma antigen (SCC) was 0.8 ng/mL (reference range: ≤ 1.5 ng/mL), carbohydrate antigen 125 (CA125) was 6.7 U/mL (reference range: 0-35 ng/mL), and pro-gastrin-releasing peptide (pro-GRP) was 24.99 pg/mL (reference range: ≤ 63 pg/mL).
Case 2: The biochemical indicators showed the following results: CEA was 3.22 ng/mL, CA125 was 9 U/mL, NSE was 11.21 ng/mL, CK19 was 1.87 ng/mL, SCC was 0.7 ng/mL, and pro-GRP was 27.65 pg/mL, all of which were normal.
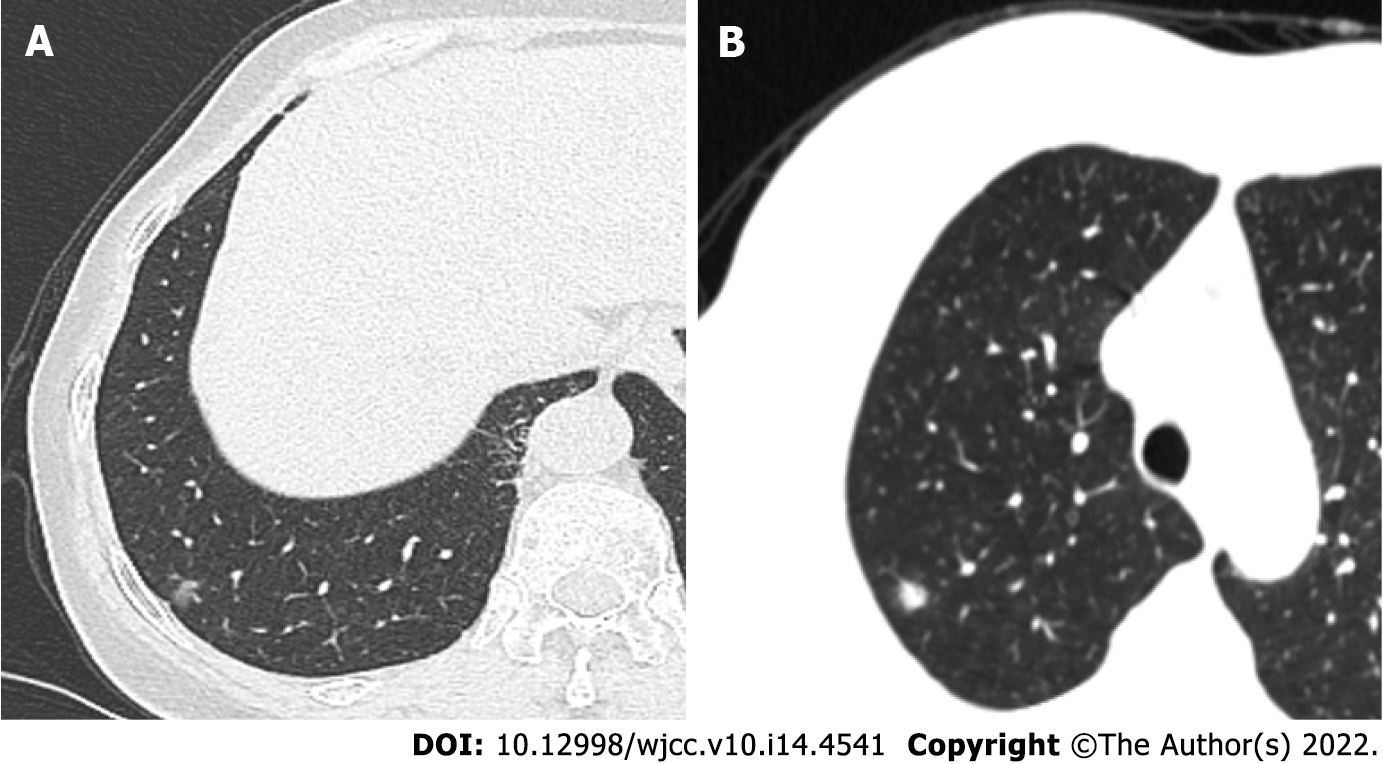
Case 1: On December 7, 2020, the findings of thoracic enhanced CT performed at our hospital revealed bronchitis, right lower pulmonary bullae, and subpleural nodules and pleural traction in the lower lobe of the right lung of the patient (Figure 1A).
Case 2: The patient underwent chest CT examination at our hospital on January 19, 2021 and was identified as having nodules in the right upper lobe of the lung (Figure 1B).
Case 1: A small subpleural nodule was found in the lower lobe of the right lung. The nodule was approximately in diameter and did not involve the visceral pleura. A wedge-shaped resection of the nodule was performed.
Case 2: After performing preoperative puncture and locating the right upper lobe nodule, a solid nodule with a diameter of 0.7 cm was palpated around the lobe. The nodule of the right upper lobe was excised by a wedge-shaped incision.
Case 1: A piece of grayish red lung tissue was removed by wedge resection; the tissue measured 9 cm × 3.5 cm × 2 cm. The pleura was grayish red and smooth; a grayish white nodule was found by multi-section incision. The nodule measured 0.6 cm × 0.5 cm × 0.3 cm. The texture of the nodule was similar to that of normal salivary glands. It showed a clear boundary attached to the surrounding normal lung tissue, which was away from the anastomosis line, and the remaining section was grayish red and soft.
Case 2: Upon gross pathological examination, we identified a piece of grayish red lung tissue measuring 10 cm × 4 cm × 2 cm. A partial incision was made by the surgeon. The pleura was grayish red and smooth; a grayish white nodule was later found upon incision. The nodule measured 0.6 cm × 0.5 cm × 0.5 cm. The texture of the nodule was similar to that of normal salivary glands. The nodule showed clear boundaries and was attached to the pleura 2 cm away from the anastomosis line. The remaining section was grayish red and soft.
Surgical specimens were fixed with 4% neutral buffer formaldehyde solution (18-24 h) and embedded with paraffin; sections (4 μm thick) were subjected to hematoxylin-eosin staining[10] and immunohistochemistry analyses.
Immunohistochemical staining: Immunohistochemical analyses were performed on paraffin-embedded sections using primary antibodies against the following proteins: P40, P63, P53, thyroid transcription factor 1, CK5/6, CD34, Ki-67, and collagen IV. All primary antibodies were purchased from Fuzhou Maixin Biotechnology Co., Ltd. (Fuzhou, China). Immunohistochemistry was performed according to the manufacturer’s instructions. Polybutylene succinate was used as a negative control. Staining was performed using the Roche Benchmark XT medical system (Shanghai).
Genetic testing: Mutations in the EGFR, ALK, ROS1, KRAS, PIK3CA, BRAF, human epidermal growth factor receptor-2 (HER2), REarranged during transfection, and neuroblastoma RAS genes were detected using the ADX Arms and the Amoydx FFPE DNA/RNA Tissue Kit (Xiamen Ade Biomedical Technology Co., Ltd.). All experimental procedures were performed strictly according to the manufacturer’s instructions.
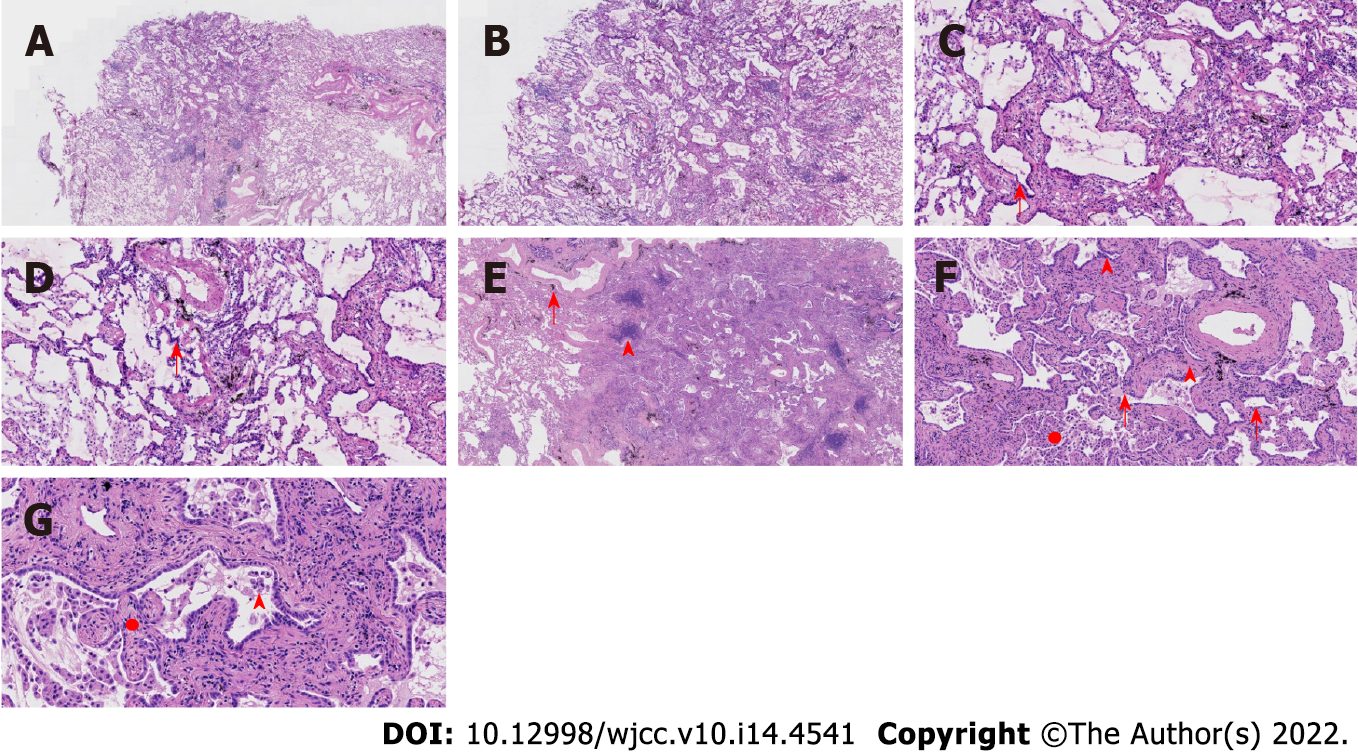
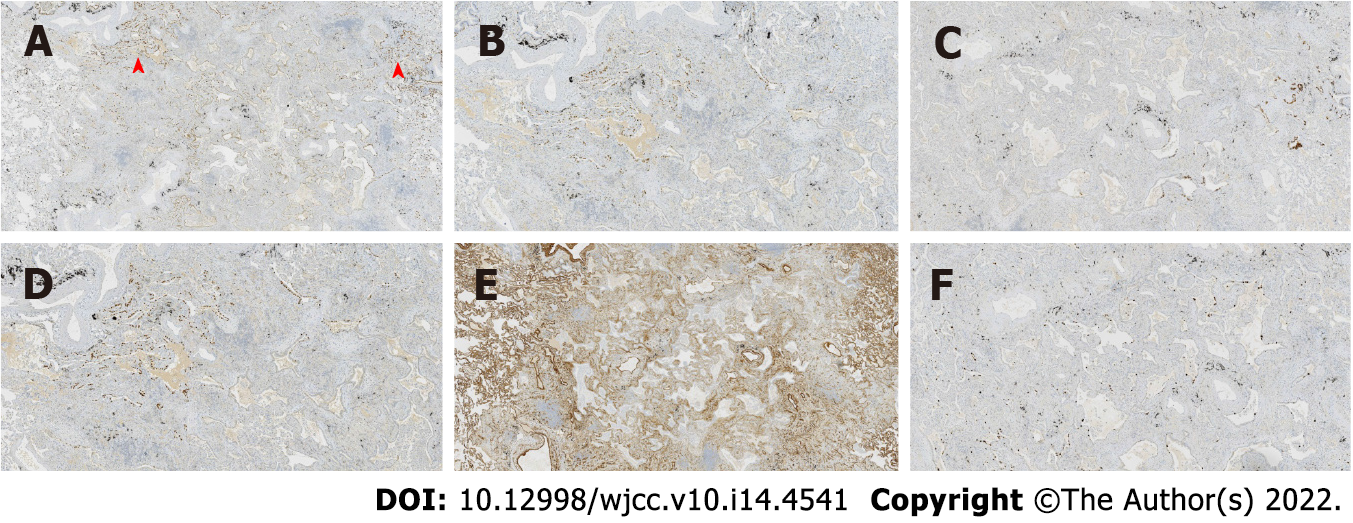
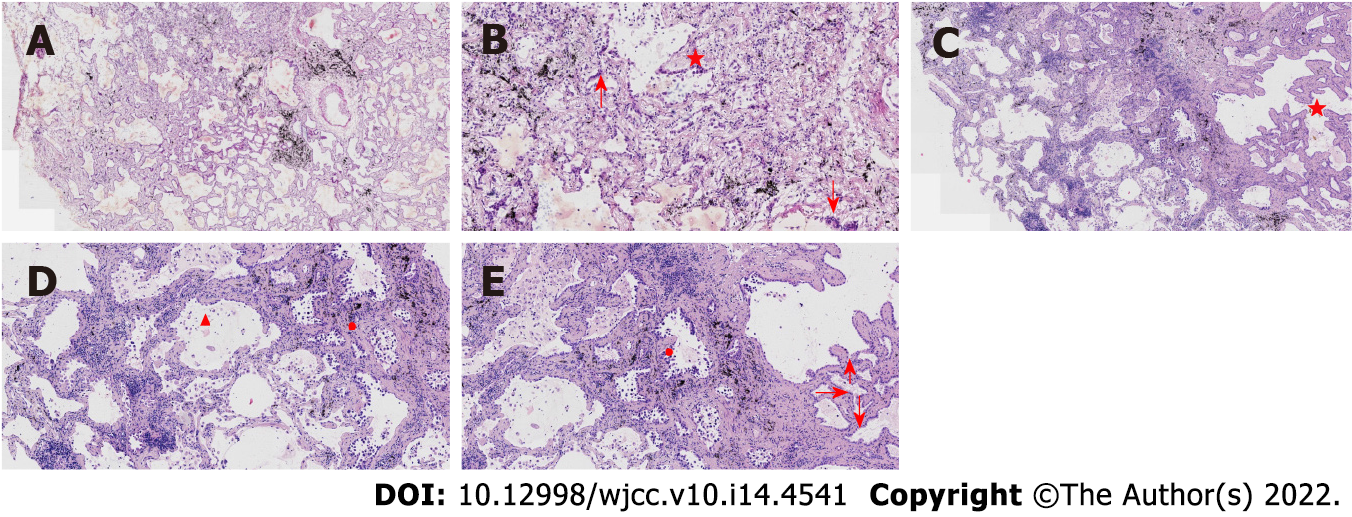
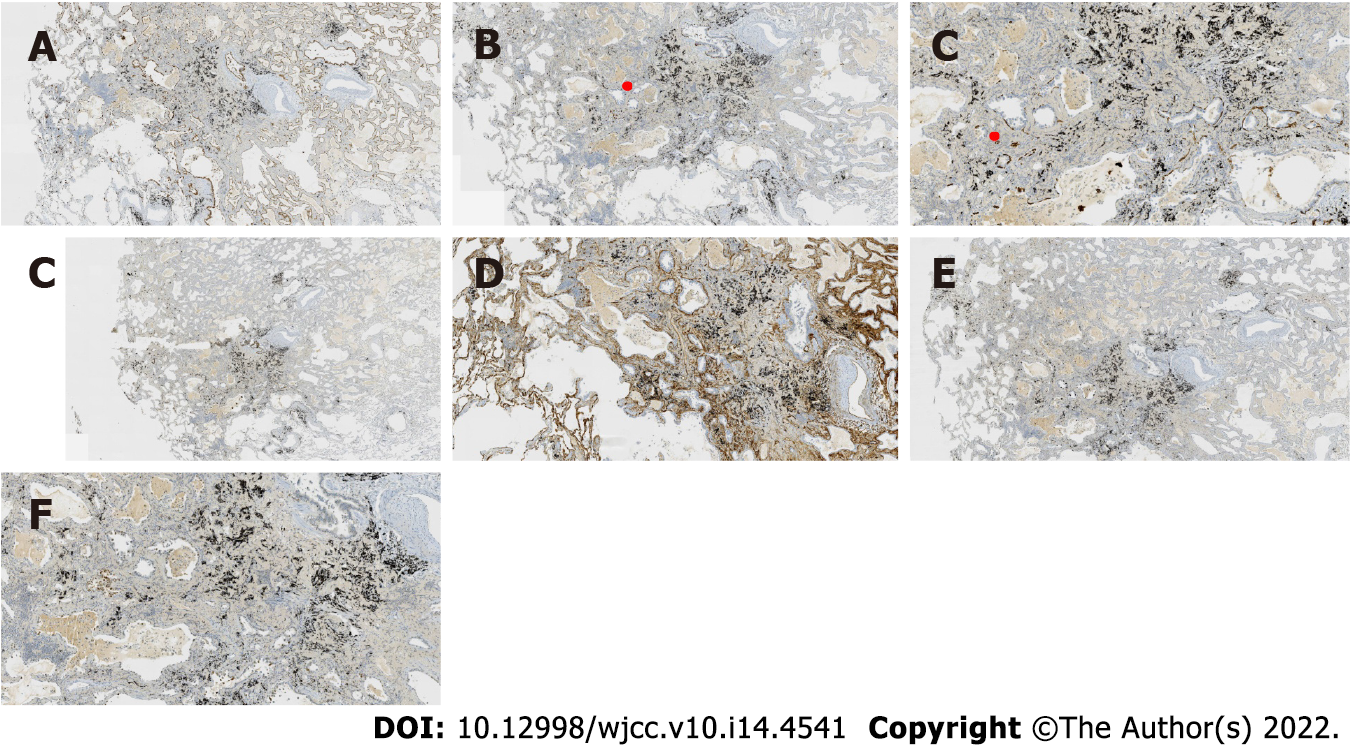
Case 1: At low magnification (100 ×), the tumor boundary was relatively clear, and air cavities were present. The pulmonary lobular artery and bronchioles were observed, and the peripheral stromal lymphocytes were localized (Figures 2A, 2B and 2E). At high magnification (200 × and 400 ×), most tumor cells were arranged in a monolayer structure, and the local part appeared as a bilayer structure. Morphologically, the cells were observed to be of medium size (the size of the nucleus and normal phagocytic nuclei was equivalent in the alveolar space); the nuclear chromatin was pale and homogeneous, and local cilia were seen (Figures 2C-G). Thyroid transcription factor 1 (TTF1) was expressed in bronchioles and the peripheral alveolar epithelium, with the only difference being in the intensity of expression. The results of P40, P63, and CK5/6 staining were the same, and staining was positive only in the bilayer structure of the tumor (Figure 3).
Case 2: At low magnification (100 ×), most cells appeared with moderate density, focal hyperplasia, and stroma within the focal lymphocytic infiltration; at high magnification (200 × and 400 ×), the tumor cells were arranged as an acinar structure and accessory wall structure; most cells were observed to be medium sized, the nuclear chromatin was pale and homogeneous, and cilia were seen. The focal nucleus was enlarged and atypical (Figure 4). TTF-1 was positive; the results for P63 and CK5/6 staining were the same, and only basal cells were seen in the hyperplasia area. CD34 was present in the alveolar structure, and the Ki-67 index was low (Figure 5).
Genetic tests were performed using the patients’ DNA samples to check for mutations in EGFR, ALK, ROS1, KRAS, PIK3CA, BRAF, HER2, RET, and NRAS genes. No gene mutations were detected in case 1, while HER2 gene mutation was detected in case 2.
Based on the histological characteristics and results of immunohistochemical staining, the two patients were diagnosed as having BA with unusual presentation.
Complete wedge resection was performed at the Thoracic Surgery Department of Liaocheng People’s Hospital.
After surgical resection, neither patient received radiotherapy or chemotherapy. At the time of writing this report, which is 11 and 12 mo postoperatively for the two patients, respectively, both of them have recovered well without signs of disease.
In 2018, BA was proposed by Chang et al[2] as a new type of lung tumor, defined as a group of pulmonary tumors that could be benign or have a potential for malignant transformation depending on the epithelial cell composition of the bronchiolar anatomy. These include classic CMPT and non-classic CMPT, which differ according to histological aspects. BAs can be further divided into proximal (similar to proximal bronchioles) and distal (similar to respiratory bronchioles) types based on the histomorphology (comparing histological features of different grades of bronchial epithelial cells and their similarity with the bronchioles) and immunohistochemical characteristics. Proximal-type BAs comprise numerous prominent mucinous cells and are well defined with ciliated cells and intact basal layer cells that are arranged in a papillary or flattened pattern. Conversely, the distal form usually shows a flattened pattern and comprises few mucinous cells, cubic cells, and/or ciliated cells. Although there is some overlap between the characteristics of the two types, some lesions may lack one or both of these components. Zheng et al[4] reported that mucinous and papillary components are usually present throughout classic CMPTs but may be absent in their “non-classic” counterparts. Furthermore, Shao et al[3] also found mixed-type BAs with monolayered lesions[2,4,11].
In this study, two very rare cases of BAs comprising mucinous cells are reported. The cell arrangement observed showed a flattened pattern, indicating the distal type of BA. Although tumor cells formed an adenoid or papillary structure, the ciliary structure could be seen locally in lumen cells. Many studies have reported that the ciliary structure in lumen cells can distinguish this type of tumor from an adenocarcinoma, which is an important characteristic to help differentiate between the two tumor types[12]. However, in the two current cases, not every lumen cell had cilia, and the basal cells could not be easily observed, thus causing some difficulties in diagnosis, particularly when the specimen was frozen. Therefore, interpretations should be made considering both atypia of cells and their arrangement. In our two cases, most cells were loosely arranged, the morphology of glandular epithelial cells was not atypical, and the cytoplasm of local cells was transparent. Few intranuclear inclusion bodies were seen under a high-power microscope; this finding, together with a marginally increased nucleoplasmic ratio, suggested that the lesion was benign.
In the second case, atypic cells and the absence of the entire lesion’s bilayer structure complicated the diagnosis. However, these lesions were different from adenocarcinoma in situ (AIS) and invasive adenocarcinoma. The tumor cells of AIS comprise type II alveolar epithelial cells and/or Clara cells, which grow along the original alveolar wall without destroying the alveolar structure. In this case, ciliated columnar cells or mucinous cells were rarely present, and cell atypia was more pronounced than that in BA. The boundary of invasive adenocarcinoma is not discernible, the alveolar structure is destroyed, and the growth is rapid. In addition, the micropapillary structure can be seen in the lumen and necrosis is visible, cell atypia is evident, and nuclear cleavage is widely observed[2-4].
Wang et al[13] considered BA as a kind of tumor associated with bronchioles, and bronchiole involvement can be found in almost all BAs. Upon careful observation, we also found the tumor to have expanded from bronchioles to the surrounding alveolar walls. Meanwhile, we also observed the pulmonary lobular artery and bronchioles in local ares in these two cases; this formed a relatively robust basis for our diagnosis.
In typical morphologic cases, the double-layer structure is obvious, and ciliary cells and mucous cells are clearly recognizable on the lumen surface, eliminating the need for immunohistochemical examination. However, in our two cases, it was difficult to judge whether the basal cells were present, thus warranting immunohistochemical staining to visualize the tissue structure and cell type. In case 1, P40, P63, and CK5/6 were detected only in local areas. Whereas in case 2, P63 and CK5/6 were expressed only in atypical cells, confounding our diagnosis. Many reports have indicated that the double-layer structure is essential in the diagnosis of BA; however, based on our understanding of the current cases and review of the related literature, we call these two lesions as monolayer BA lesions[14,15].
The presence of cellular atypia and the lack of the basal cell layer in monolayer BA lesions suggest their potential to transform into malignant tumors. These findings may reflect the continuous malignant transformation process of benign adenomas of the bronchial epithelium. Further large-scale studies of similar cases are required to investigate whether monolayer BA lesions are accompanied by atypical bronchiolar epithelial hyperplasia, whether they are precancerous lesions and are similar to the atypical hyperplasia of the breast, and whether they will eventually become AIS or even invasive adenocarcinoma[15].
Although the distal type of bronchial adenoma typically has cilia and can be found to extend with normal bronchioles, these characteristics are not easy to observe on intraoperative frozen sections[6,16,17]. The evaluation of the differentiation of bronchial adenoma and cancer requires immunohistochemistry-assisted diagnosis, which is not currently performed during the operation. Therefore, performing a differential diagnosis of bronchial adenoma and carcinoma using intraoperative frozen sections during operation is difficult and challenging.
Although some studies have reported that ill-defined peripheral opacity and pseudocavities of a ground-glass lung nodule on CT differentiate BA from AIS or minimally invasive adenocarcinoma[18], these aspects are not absolute. Thus, they provide some hints, but more comprehensive findings are required for differentiation of these lesions.
Kamata et al[19] identified cancer-driving gene mutations in CMPT, supporting the notion that these lesions are neoplastic rather than reactive or metaplastic. Unlike previous studies that primarily focused on EGFR and BRAF genes[5-9,20], we evaluated nine genes associated with susceptibility to BAs. Case 1 was negative for mutations in all genes. In case 2, HER2 gene mutation was found. Given the small number of samples in this case report, the significance of HER2 gene mutation needs to be further studied in a larger number of samples.
The results of our two cases are shown in Table 1. Although no meaningful conclusions could be drawn, these findings encourage further work using a larger sample size with control cases for better comparison. The current two cases have monolayer BA lesions. Some unresolved questions remain to be answered to determine whether the lesion is a benign adenoma or part of the process of malignant transformation from benign adenoma of the bronchial epithelium. Furthermore, whether the lesions with atypical bilayer structures are similar to atypical hyperplastic lesions of the breast remains to be elucidated. In addition, whether these lesions can be called atypical BA and whether they are invasive precursor lesions need to be evaluated. Finally, future studies should examine whether HER2 gene mutation has diagnostic significance as a prognostic indicator in BA.
| Cases characteristic | Previous bronchiolar adenoma | Case 1 | Case 2 |
| Cellular atypia | No | No | Local atypical |
| Immunohistochemistry (basal cell display) | Constant continuous existence | Basal cells mostly absent | Basal cells mostly absent; present in cellular atypia |
| Genetic testing | EGFR and BRAF gene mutations | None | HER2 gene mutation |
Regardless of whether BA is benign or potentially malignant, simple surgical resection is the best choice for patient management. However, to determine BA, it is very important to use intraoperative frozen sections. Performing a differential diagnosis of bronchial adenoma and carcinoma using intraoperative frozen sections while the operation is underway is difficult and challenging. Hence, further study of this disease with a larger sample size and controls is required to draw meaningful conclusions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Neninger E, Cuba; Rioja Viera PE, Peru S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | IASLC News. The New WHO Classification of Lung Tumors. [cited 15 July 2021]. Available from: https://www.ilcn.org/the-new-who-classification-of-lung-tumors/. [Cited in This Article: ] |
| 2. | Chang JC, Montecalvo J, Borsu L, Lu S, Larsen BT, Wallace WD, Sae-Ow W, Mackinnon AC, Kim HR, Bowman A, Sauter JL, Arcila ME, Ladanyi M, Travis WD, Rekhtman N. Bronchiolar Adenoma: Expansion of the Concept of Ciliated Muconodular Papillary Tumors With Proposal for Revised Terminology Based on Morphologic, Immunophenotypic, and Genomic Analysis of 25 Cases. Am J Surg Pathol. 2018;42:1010-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Shao K, Wang Y, Xue Q, Mu J, Gao Y, Wang B, Zhou L, Gao S. Clinicopathological features and prognosis of ciliated muconodular papillary tumor. J Cardiothorac Surg. 2019;14:143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Zheng Q, Luo R, Jin Y, Shen X, Shan L, Shen L, Hou Y, Li Y. So-called "non-classic" ciliated muconodular papillary tumors: a comprehensive comparison of the clinicopathological and molecular features with classic ciliated muconodular papillary tumors. Hum Pathol. 2018;82:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Kamata T, Sunami K, Yoshida A, Shiraishi K, Furuta K, Shimada Y, Katai H, Watanabe S, Asamura H, Kohno T, Tsuta K. Frequent BRAF or EGFR Mutations in Ciliated Muconodular Papillary Tumors of the Lung. J Thorac Oncol. 2016;11:261-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Chu HH, Park SY, Cha EJ. Ciliated muconodular papillary tumor of the lung: The risk of false-positive diagnosis in frozen section. Hum Pathol Case Rep. 2017;7:8-10. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Kim L, Kim YS, Lee JS, Choi SJ, Park IS, Han JY, Kim JM, Chu YC. Ciliated muconodular papillary tumor of the lung harboring BRAF V600E mutation and p16INK4a overexpression without proliferative activity may represent an example of oncogene-induced senescence. J Thorac Dis. 2017;9:E1039-E1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Liu L, Aesif SW, Kipp BR, Voss JS, Daniel S, Aubry MC, Boland JM. Ciliated Muconodular Papillary Tumors of the Lung Can Occur in Western Patients and Show Mutations in BRAF and AKT1. Am J Surg Pathol. 2016;40:1631-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Udo E, Furusato B, Sakai K, Prentice LM, Tanaka T, Kitamura Y, Tsuchiya T, Yamasaki N, Nagayasu T, Nishio K, Fukuoka J. Ciliated muconodular papillary tumors of the lung with KRAS/BRAF/AKT1 mutation. Diagn Pathol. 2017;12:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Kataoka T, Okudela K, Matsumura M, Mitsui H, Suzuki T, Koike C, Sawazumi T, Umeda S, Tateishi Y, Yamanaka S, Ishikawa Y, Arai H, Tajiri M, Ohashi K. A molecular pathological study of four cases of ciliated muconodular papillary tumors of the lung. Pathol Int. 2018;68:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Chuang HW, Liao JB, Chang HC, Wang JS, Lin SL, Hsieh PP. Ciliated muconodular papillary tumor of the lung: a newly defined peripheral pulmonary tumor with conspicuous mucin pool mimicking colloid adenocarcinoma: a case report and review of literature. Pathol Int. 2014;64:352-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Song L, Ye Y, Li W. Long Noncoding RNA DIO3OS Hinders Cell Malignant Behaviors of Hepatocellular Carcinoma Cells Through the microRNA-328/Hhip Axis. Cancer Manag Res. 2020;12:3903-3914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Wang EH. Bronchiolar adenoma: a benign tumor easily confused with cancer. Chin J Pathol. 2019;48 (6):425-432. [DOI] [Cited in This Article: ] |
| 14. | Shao J, Yin JC, Bao H, Zhao R, Han Y, Zhu L, Wu X, Shao Y, Zhang J. Morphological, immunohistochemical, and genetic analyses of bronchiolar adenoma and its putative variants. J Pathol Clin Res. 2021;7:287-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Shao JC, Han YC. Some problems in pathological diagnosis of bronchiolitis adenoma. Chin J Pathol. 2020;49 (6):529-533. [DOI] [Cited in This Article: ] |
| 16. | Han X, Hao J, Ding S, Wang EH, Wang L. Bronchiolar Adenoma Transforming to Invasive Mucinous Adenocarcinoma: A Case Report. Onco Targets Ther. 2021;14:2241-2246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Shirsat H, Zhou F, Chang JC, Rekhtman N, Saqi A, Argyropoulos K, Azour L, Simms A, Melamed J, Hung YP, Roden AC, Mino-Kenudson M, Moreira AL, Narula N. Bronchiolar Adenoma/Pulmonary Ciliated Muconodular Papillary Tumor. Am J Clin Pathol. 2021;155:832-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Cao L, Wang Z, Gong T, Wang J, Liu J, Jin L, Yuan Q. Discriminating between bronchiolar adenoma, adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung with CT. Diagn Interv Imaging. 2020;101:831-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Kamata T, Yoshida A, Kosuge T, Watanabe S, Asamura H, Tsuta K. Ciliated muconodular papillary tumors of the lung: a clinicopathologic analysis of 10 cases. Am J Surg Pathol. 2015;39:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Gao H, Du XL, Chen CN, Song GX, Gu YL, Li HX. [Bronchiolar adenoma: a clinicopathological analysis of 15 cases]. Zhonghua Bing Li Xue Za Zhi. 2020;49:556-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |