Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4414
Peer-review started: November 21, 2021
First decision: December 9, 2021
Revised: December 19, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: May 16, 2022
To ensure clinical efficacy and prolong patient survival, treatments such as surgery and microwave ablation (MWA) are used for early liver cancer. MWA is preferred because it effectively preserves the normal liver tissue and causes transient coagulation necrosis of local liver tumor cells. However, due to technical limitations, the cancerous liver tissue cannot be completely ablated; therefore, the probability of local tumor recurrence is high.
To investigate the clinical efficacy and safety of ultrasound-guided percutaneous MWA in the treatment of small liver cancer.
A total of 118 patients treated for small liver cancer in The Central Hospital of Yongzhou from January 2018 to April 2019 were selected. Sixty-six patients received ultrasound-guided percutaneous MWA (MWA group) and 52 received laparoscopic surgery (laparoscope group). The operation time, blood loss, hospital stay, and medical expenses of both groups were statistically analyzed. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), alpha fetal protein (AFP), carcinoembryonic antigen (CEA), and peripheral blood regulatory T lymphocytes (Treg) levels were evaluated pre- and post-operatively. The cross-sectional area of tumors measured before and after ablation was analyzed statistically; the therapeutic effect was compared between both groups in terms of surgical complications, 2-year progression-free survival rate, and overall survival rate.
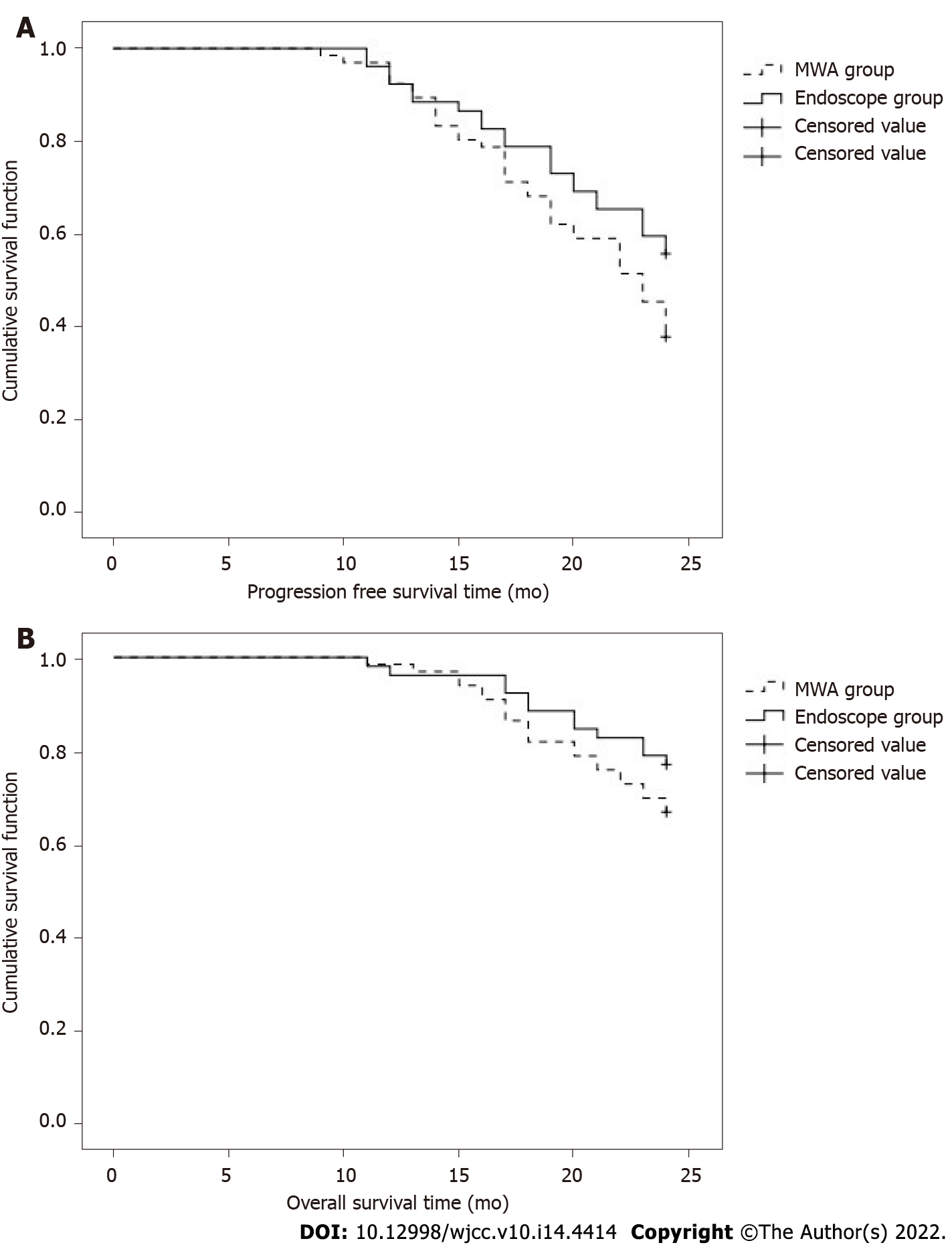
The operation time, blood loss, hospital stay, and medical expenses in the MWA group were lower than those of the laparoscope group, and the differences were significant (P < 0.05); these parameters, and ALT, AST, TBIL, and ALB levels were compared preoperatively between both groups, and there was no significance (P > 0.05). The operation time, blood loss, hospital stay, and medical expenses for 2 d and 1 wk after surgery, the ALT and AST of the MWA group were lower than those of the laparoscope group, and the difference was significant (P < 0.05). The operation time, blood loss, hospital stay, and medical expenses, and serum AFP, CEA, and Treg levels were measured preoperatively and 4 and 8 wk postoperatively, and there were no significant differences between the two groups (P > 0.05). Compared with preoperative levels, serum AFP, CEA, and Treg levels in both groups were decreased (P < 0.05). The lesion in the MWA group had a maximum area of 4.86 ± 0.90 cm2, 1.24 ± 0.57 cm2, and 0.31 ± 0.11 cm2 preoperatively, 1 and 3 mo postoperatively, respectively. Fifty-eight of them achieved complete response and eight achieved a partial response. After 2 years of follow-up, the progression-free and overall survival rates in the MWA group were 37.88% and 66.67%, respectively, compared with 44.23% and 76.92% in the laparoscope group, with no significant difference (P > 0.05).
The effects of ultrasound-guided percutaneous MWA in the treatment of small liver cancer are similar to those of laparoscopic surgery. However, ablation causes less trauma and liver dysfunction.
Core Tip: Through a set of controls, it was confirmed that the effect of ultrasound-guided percutaneous microwave ablation in the treatment of small liver cancer is similar to that of laparoscopic surgery. However, ablation will also cause less trauma and liver dysfunction, so there is more room for development in clinical applications.
- Citation: Zhong H, Hu R, Jiang YS. Evaluation of short- and medium-term efficacy and complications of ultrasound-guided ablation for small liver cancer. World J Clin Cases 2022; 10(14): 4414-4424
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4414.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4414
Liver cancer is a common malignancy in oncology, with a high incidence and mortality; primary liver cancer is the main clinical tumor[1]. The specific cause of liver cancer is still unclear but long-term studies have found that it may be related to cirrhosis and viral hepatitis[1]. In order to ensure clinical efficacy and prolong patient survival, radical treatments such as surgery and microwave ablation (MWA) are used for early liver cancer, while local chemoradiotherapy combined with systemic therapy is used for advanced liver cancer[2]. MWA is a rapidly developing interventional therapy technique for liver cancer; it is safe, reliable, well-tolerated, and has few postoperative complications[3]. The main principle of MWA is the puncture of the central area of the tumor (through the skin) using a special microwave needle. When the tissue is treated using microwaves, it absorbs a large amount into itself, resulting in rapid production of a high amount of heat (up to 100 ℃ instantly). Next to surgery and liver transplantation, it is the preferred method because it effectively preserves the normal liver tissue and causes transient coagulative necrosis of local liver tumor cells due to hyperpyrexia; it may also increase the immunity of the body[4]. However, due to technical limitations, the cancerous liver tissue cannot be completely ablated; therefore, the probability of local tumor recurrence is high[5]. Presently, MWA for liver cancer has a variety of guidance methods, including laparoscopy, computed tomography (CT), ultrasound, intraoperative methods, etc. Ultrasound-guided MWA for primary liver cancer is widely used[6]. This study aimed to investigate the clinical efficacy and safety of ultrasound-guided percutaneous MWA in the treatment of small liver cancer.
A total of 118 patients with small liver cancer treated at The Central Hospital of Yongzhou of Hunan Province from January 2018 to December 2020 were selected for this study. Sixty-six patients underwent ultrasound-guided percutaneous MWA (MWA group) and 52 underwent laparoscopic surgery (laparoscope group).
Inclusion criteria: (1) Patients aged 42 to 79 years; (2) primary liver cancer diagnosed based on the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2019 Edition)[7,8]; (3) maximum lesion diameter less than 3.0 cm, and disease confirmed by CT, magnetic resonance imaging, or liver puncture examination; (4) no distant metastasis to bile duct or vessels found in pre-treatment examination; (5) all patients who received treatment diagnosed for the first time; and (6) those with provided informed consent.
Exclusion criteria: Those (1) Diagnosed with other malignant tumors; (2) with Child-Pugh classification Grade C; (3) who had abdominal cavity and hepatobiliary operations within the past 6 mo; (4) with a history of acute myocardial infarction or cerebrovascular disease within the past 6 mo; (5) experiencing blood coagulation abnormalities, blood system diseases, serious infections, etc.; and (6) with other treatment contraindications.
The patient was placed in the supine position. After successful local anesthesia, routine disinfection and towel laying were performed. Conventional color Doppler and contrast-enhanced ultrasound were performed again to determine the puncture point and angle and depth of needle insertion. An MWA needle was used under the guidance of color Doppler ultrasound according to the predetermined puncture path and depth. The MWA needle was inserted into the edge of the tumor area. According to the diameter of the tumor, the best location of the electrode should be the site where the tumor and surrounding normal tissue can be thermally coagulated and necrotized to within at least 1 cm. Different microwave programs and powers can be selected according to the size of the tumor for thermal coagulation necrosis. Once the MWA needle reached the puncture edge of the tumor, the microwave device was activated. The MWA time was about 8–12 min, after which the cold circulation system was turned off. After ablation, needle ablation was performed. Patients with cancer less than 3.0 cm in diameter can be treated with one-needle multi-point MWA and those with cancer greater than 3.0 cm in diameter with multi-needle multi-point MWA. The changes in tumor tissue during MWA can be monitored in real-time. Hemostatic drugs, analgesics, sedatives, and fluids can be administered intravenously if necessary[8].
The patient was placed in the supine position and under general anesthesia, a 1-cm incision was made under the umbilical cord. After establishing pneumoperitoneum, a laparoscope was inserted to explore the exact location, the number of the mass(es), and the surrounding blood vessels. The ligaments around the liver were removed with the ultrasonic knife and the liver was mobilized. The excision line was marked 2 cm away from the tumor margin, and the liver tissue was removed. Titanium clips were used whenever large vessels were encountered during excision. If the liver section appeared to be bleeding, bipolar electrocoagulation was used to stop the bleed. After resecting the tumor and confirming that there was no active bleeding in the abdominal cavity, the laparoscopic instrument was withdrawn, the air in the abdominal cavity was discharged, and the incision sutured layer by layer.
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), alpha fetal protein (AFP), carcinoembryonic antigen (CEA), and peripheral blood regulatory T lymphocytes (Treg) levels were evaluated before and after the surgery. The cross-sectional area of tumors measured before and after ablation was analyzed statistically, and the therapeutic effect in terms of surgical complications and 2-year progression-free and overall survival rates were compared between the two groups.
Five mL of venous blood was extracted in the fasting state, centrifuged at 3000 r/min for 5 min, and the serum was separated. AFP and CEA were detected using enzyme-linked immunoassay. Serum ALT, AST, TBIL, and ALB levels were detected in the laboratory to evaluate the liver function in the patients. FACSCalibur flow cytometry (Bectond-Dickinson, USA) was used to detect peripheral blood regulatory T cells.
According to RECIST efficacy evaluation criteria[9,10] for solid tumors, they can be divided into complete response (CR), partial response (PR), stable disease, and progressive disease.
The two groups of patients were followed up for 3 years by return visits or via telephone. The follow-up ended in April 2021, and patient survival rates in both groups were recorded.
In this study, the serum ALT, AST, TBIL, and ALB levels in the patients were tested by normal distribution test, and were in line with the approximate normal distribution or normal distribution, represented by mean ± SD; t-test was used for comparison between groups. Enumeration data (gender, smoking, drinking, Child-pugh, etc.) were compared by χ2 test and expressed as percentages. The Kaplan-Meier method was used for survival analysis, and the log-rank test was used for the comparison of survival time. Professional SPSS 21.0 software was used for data processing, and the test level was α = 0.05.
There were no significant differences in age, body mass index, sex, smoking, alcohol consumption, and the number of lesions between the MWA and endoscopic groups (P > 0.05), as shown in Table 1.
| Basic information | MWA group (n = 66) | Laparoscope group (n = 52) | t/χ2 | P value |
| Age (yr) | 58.7 ± 8.6 | 57.3 ± 9.1 | 0.856 | 0.394 |
| BMI (kg/m2) | 24.1 ± 2.0 | 23.8 ± 2.2 | 0.774 | 0.440 |
| Lesion diameter (cm2) | 2.13 ± 0.48 | 2.01 ± 0.44 | 1.398 | 0.165 |
| Number of lesions | 2.14 ± 0.50 | 2.03 ± 0.48 | 1.207 | 0.230 |
| Gender | 0.952 | 0.329 | ||
| Male | 36 (54.55) | 33 (63.46) | ||
| Female | 30 (45.45) | 19 (36.54) | ||
| Smoking | 1.187 | 0.276 | ||
| Yes | 24 (36.36) | 14 (26.92) | ||
| No | 42 (63.64) | 38 (73.08) | ||
| Alcohol | 2.638 | 0.104 | ||
| Yes | 22 (33.33) | 25 (48.08) | ||
| No | 44 (66.67) | 27 (51.92) | ||
| Child–pugh | 2.336 | 0.126 | ||
| Class A | 30 (45.45) | 31 (59.62) | ||
| Class B | 36 (54.55) | 21 (40.38) | ||
| History of hepatitis B | 1.291 | 0.256 | ||
| Yes | 39 (59.09) | 36 (69.23) | ||
| No | 27 (40.91) | 16 (30.77) | ||
| AFP (ng/mL) | 0.968 | 0.325 | ||
| ≤ 20 | 22 (33.33) | 13 (25.00) | ||
| >20 | 44 (66.67) | 39 (75.00) |
The operation time, blood loss, hospital stay, and medical expenses in the MWA group were lower than those in the laparoscope group, and the differences were significant (P < 0.05, Table 2).
| Group | n | Operation time (min) | Blood loss (mL) | Hospital stays (d) | Medical expenses (Thousand Yuan) |
| MWA group | 66 | 55.81 ± 9.64 | 8.94 ± 2.26 | 3.3 ± 0.8 | 2.28 ± 0.21 |
| Laparoscope group | 52 | 62.30 ± 10.57 | 22.83 ± 6.18 | 5.1 ± 1.3 | 2.91 ± 0.30 |
| t value | -3.479 | -16.897 | -9.249 | -13.401 | |
| P value | 0.001 | 0.000 | 0.000 | 0.000 |
The operation time, blood loss, hospital stay, medical expenses, and ALT, AST, TBIL, and ALB levels in the MWA and laparoscope groups before surgery were compared, and there was no significance (P > 0.05). The operation time, blood loss, hospital stay, medical expenses 2 and 7 d after surgery, and ALT and AST levels in the MWA group were lower than those in the laparoscope group, and the difference was significant (P < 0.05, Table 3).
| Index | Before surgery | 2 d after surgery | 1 wk after surgery |
| ALT (U/L) | |||
| MWA group (n = 66) | 32.5 ± 7.0 | 175.3 ± 36.8a | 57.1 ± 10.4a |
| Laparoscope group (n = 52) | 34.1 ± 6.7 | 193.0 ± 40.2a | 66.5 ± 12.1a |
| t value | -1.256 | -2.490 | -4.535 |
| P value | 0.212 | 0.014 | 0.000 |
| AST (U/L) | |||
| MWA group (n = 66) | 34.9 ± 8.6 | 193.7 ± 33.2a | 64.2 ± 15.0a |
| Laparoscope group (n = 52) | 32.6 ± 7.3 | 218.0 ± 41.8a | 76.7 ± 16.3a |
| t value | 1.540 | -3.520 | -4.326 |
| P value | 0.126 | 0.001 | 0.000 |
| TBIL (μmol/L) | |||
| MWA group (n = 66) | 15.9 ± 3.0 | 36.8 ± 9.2a | 24.1 ± 6.5a |
| Laparoscope group (n = 52) | 16.3 ± 4.1 | 39.5 ± 9.8a | 26.4 ± 7.3a |
| t value | -0.612 | -1.538 | -1.807 |
| P value | 0.542 | 0.127 | 0.073 |
| ALB (g/L) | |||
| MWA group (n = 66) | 39.8 ± 3.1 | 35.0 ± 3.4a | 37.8 ± 2.8a |
| Laparoscope group (n = 52) | 41.0 ± 4.4 | 34.2 ± 3.1a | 36.9 ± 3.1a |
| t value | -1.736 | 1.319 | 1.653 |
| P value | 0.085 | 0.190 | 0.101 |
The operation time, blood loss, hospital stay, and medical expenses, and serum AFP, CEA, and Treg levels were measured preoperatively and at 4 and 8 wk after the surgery, and there were no significant differences between the two groups (P > 0.05). Compared with preoperative levels, serum AFP, CEA, and Treg levels in both groups were decreased (P < 0.05), as shown in Table 4.
| Index | Before surgery | 4 wk after surgery | 8 wk after surgery |
| AFP (ng/mL) | |||
| MWA group (n = 66) | 98.6 ± 20.5 | 43.2 ± 14.3a | 15.1 ± 4.6a |
| Laparoscope group (n = 52) | 103.8 ± 25.1 | 39.7 ± 13.6a | 13.6 ± 4.8a |
| t value | -1.239 | 1.349 | 1.725 |
| P value | 0.218 | 0.180 | 0.087 |
| CEA (ng/mL) | |||
| MWA group (n = 66) | 14.82 ± 4.16 | 7.18 ± 2.30a | 2.29 ± 0.77a |
| Laparoscope group (n = 52) | 16.33 ± 5.28 | 8.01 ± 2.57a | 2.56 ± 0.81a |
| t value | -1.738 | -1.848 | -1.848 |
| P value | 0.085 | 0.067 | 0.067 |
| Treg (%) | |||
| MWA group (n = 66) | 9.53 ± 2.33 | 6.81 ± 1.53a | 5.52 ± 1.00a |
| laparoscope group (n = 52) | 10.04 ± 2.56 | 7.41 ± 1.76a | 5.80 ± 1.43a |
| t value | -1.130 | -1.979 | -1.250 |
| P value | 0.261 | 0.050 | 0.214 |
The lesion in the MWA group had a maximum area of 4.86 ± 0.90 cm2, 1.24 ± 0.57 cm2, and 0.31 ± 0.11 cm2 before, at 1, and 3 mo after the surgery, respectively. Among them, 58 and eight patients achieved CR PR, respectively.
After 2 years of follow-up, the progression-free and overall survival rates of patients in the MWA group were 37.88% and 66.67%, respectively, compared with 44.23% and 76.92% for those in the laparoscope group, respectively, with no significant difference (P > 0.05, Table 5, Figure 1A, and B).
| Group | n | Progression-free survival | Overall survival |
| MWA group | 66 | 25 (37.88) | 44 (66.67) |
| Laparoscope group | 52 | 23 (44.23) | 40 (76.92) |
| χ2 | 0.486 | 1.492 | |
| P value | 0.486 | 0.222 |
There was no significant difference in the complication rate between the MWA (4.55%) and laparoscope (9.62%) groups (P > 0.05), as shown in Table 6.
| Group | n | Abdominal infection | Gastrointestinal bleeding | Upper gastrointestinal hemorrhage | Complication rate |
| MWA group | 66 | 2 | 0 | 1 | 3 (4.55) |
| Laparoscope group | 52 | 3 | 2 | 0 | 5 (9.62) |
| χ2 | 1.183 | ||||
| P value | 0.277 |
These are presented in Figure 2.
Based on previous studies, this study evaluated the efficacy of ultrasound-guided MVA and traditional surgery in the treatment of small liver cancer and compared the level of postoperative liver function recovery and the incidence of complications, so as to comprehensively and objectively evaluate the advantages and disadvantages of radiofrequency ablation.
During the treatment of liver cancer, the effects on liver function should be minimized. With the development of imaging, laparoscopy, and radiofrequency electrode technology, the application of minimally invasive surgeries, such as laparoscopic hepatectomy and MWA, in clinical practice, has increased. With its advantages of accuracy and efficacy, it has become the mainstay in the treatment of primary liver cancer[11]. MWA is a commonly used non-surgical treatment in clinical practice, which has a good targeting ability and can directly act on the tumor cells in patients[12]. Ultrasonography can determine the boundary and diameter of the tumor, and clearly show the perfusion situation of the tumor. It also helps to distinguish the tumor from the gallbladder, diaphragm, intestine, etc.; it can be used for radiofrequency ablation for providing an important reference for the path, range, and angle of radiofrequency needle insertion, effectively reducing heat damage to the surrounding organs[13,14]. MWA is key in the successful treatment of liver cancer. Under the guidance of contrast-enhanced ultrasound, a predetermined preoperative ablation plan and a reasonable needle insertion method should be formulated according to the tumor size, so as to achieve the goal of complete tumor ablation with the least number of points[3,8,13,15,16].
The results of this study showed that the operation time, blood loss, hospital stay, and medical cost of the MWA group were significantly lower than those of the endoscopic group (P < 0.05). This is mainly because ultrasound-guided percutaneous MWA does not need to remove the tumor, but rather relies on the alternating current of the needle electrode to cause high-frequency movement of tissue cells and raise the local tissue temperature. The goal of tumor eradication can be achieved by causing coagulation inactivation or irreversible damage to tumor tissue, and high temperature can also disconnect the tumor from surrounding blood vessels[17,18]. Reducing the tumor tissue leads to its complete necrosis, thus reducing the chance of distant metastasis[19]. Compared with preoperative levels, serum ALT, AST, AFP, CEA, and Treg levels in both groups were significantly decreased postoperatively (P < 0.05). These results suggest that MWA can effectively control the disease by restoring the normal range of liver function and inhibiting the invasion of the liver by tumor cells. It also indicates that ultrasound-guided radiofrequency ablation can improve liver function indices and reduce the proportion of Treg cells in patients with liver cancer. This could be due to the fact that proliferation and invasion of cancer cells in patients undergoing MWA can be inhibited after treatment, thus restoring liver function. Peripheral blood Treg cells are presently the focus of clinical research. It is generally believed that Treg cell levels can reflect the prognosis of patients with malignancies, with low levels often representing a good prognosis.
The study showed that in the MWA group, the maximum cross-sectional area of the lesion was 1.24 ± 0.57 cm2 and 0.31 ± 0.11 cm2 at 1 and 3 mo after surgery, respectively. Among them, 58 and eight patients achieved CR and PR, respectively. After 2 years of follow-up, there was no significant difference in survival rate between the two groups (P > 0.05). It is suggested that the effect of ultrasound-guided percutaneous MWA is similar to that of laparoscopic surgery in the treatment of primary liver cancer. Studies have shown that MWA is effective for liver cancer patients, who need liver protection treatment and cannot tolerate surgery, and not only achieves this purpose but also improves the quality of life of patients[20-22]. The complication rate in the MWA group was lower than that in the endoscopic group, and the difference was not significant (P > 0.05), indicating that during the actual operation, the choice should be made according to the specific situation of the tumor. MWA has higher requirements for operators. If the tumor is close to the blood vessels, it could easily cause blood vessel damage. If the tumor is located on the surface of the liver, in order to reduce damage to the liver, the trajectory of the needle insertion should be deviated. Low accuracy of needle insertion leads to high tumor residue, and the effect of laparoscopic hepatectomy in such a condition is better[23]. At the same time, if the tumor is located in the center of the liver parenchyma, MWA should be chosen to avoid excessive damage to normal liver tissue and function. However, the treatment methods of the patients in this study were chosen under the advice of the attending physicians and did not consider the actual clinical situation, which needs further discussion.
Based on previous studies, this study evaluated the efficacy of ultrasound-guided microwave ablation and traditional surgery in the treatment of small liver cancer[13,24-32]. There are some limitations of this study. Due to the lack of long-term follow-up and small sample size, there is a need for further studies with larger sample size and longer follow-up. A multi-center study should be carried out to provide more detailed and reliable data on the application of ultrasound-guided MWA.
There is no significant difference between the effect of ultrasound-guided percutaneous MWA and laparoscopy in the treatment of small liver cancer, but the degree of trauma caused by MWA is small and liver function is mildly affected.
Early-stage liver cancer is often treated with surgery and microwave ablation (MWA). MWA effectively preserves normal liver tissue and causes transient coagulation and necrosis of local liver tumor cells. However, due to technical limitations, cancerous liver tissue cannot be completely ablated; therefore, the probability of local tumor recurrence is high.
Improve the clinical efficacy and safety of percutaneous MWA in the treatment of small liver cancer.
This study aimed to investigate the clinical efficacy and safety of ultrasound-guided percutaneous MWA in the treatment of small liver cancer.
A total of 118 patients treated for small liver cancer in The Central Hospital of Yongzhou from January 2018 to April 2019 were selected.
The operation time, blood loss, hospitalization time and medical expenses in the MWA group were lower than those in the laparoscopic group. The operation time, blood loss, hospitalization time, medical expenses, alanine aminotransferase and aspartate aminotransferase of the MWA group were lower than those of the laparoscopic group at 2 d and 1 wk after surgery. Compared with preoperatively, the serum alpha fetal protein, carcinoembryonic antigen and Treg levels of the two groups were reduced. The maximum area of the lesion in the MWA group was 4.86 ± 0.90 cm2, 1.24 ± 0.57 cm2, and 0.31 ± 0.11 cm2 before operation, 1 and 3 mo after operation, respectively. Of these, 58 people achieved a complete response, and 8 achieved a partial response. After 2 years of follow-up, the progression-free survival rate and overall survival rate of the MWA group were 37.88% and 66.67%, respectively, and the laparoscopic group were 44.23% and 76.92%, the difference was not statistically significant.
The effect of ultrasounds-guided percutaneous MWA in the treatment of small liver cancer are similar to those of laparoscopic surgery. However, ablation causes less trauma and liver dysfunction.
It can improve the clinical efficacy and safety of ultrasound-guided percutaneous MWA in the treatment of small liver cancer in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gall TMH, United Kingdom; Lachenmayer A, Switzerland S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Sciarra A, Park YN, Sempoux C. Updates in the diagnosis of combined hepatocellular-cholangiocarcinoma. Hum Pathol. 2020;96:48-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Keane FK, Hong TS. Role and Future Directions of External Beam Radiotherapy for Primary Liver Cancer. Cancer Control. 2017;24:1073274817729242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Hartley-Blossom Z, Alam M, Stone J, Iannuccilli J. Microwave Ablation in the Liver: An Update. Surg Technol Int. 2020;37:72-78. [PubMed] [Cited in This Article: ] |
| 4. | Xu Z, Xie H, Zhou L, Chen X, Zheng S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:656-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Li S, Shi S, Li A, Liu H, Cai L. Diffusion-Weighted Magnetic Resonance Imaging in Assessment of Primary Liver Cancer after HIFU Treatment. J Coll Physicians Surg Pak. 2019;29:305-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 44] [Reference Citation Analysis (0)] |
| 8. | Zhu F, Rhim H. Thermal ablation for hepatocellular carcinoma: what's new in 2019. Chin Clin Oncol. 2019;8:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2583] [Cited by in F6Publishing: 2936] [Article Influence: 209.7] [Reference Citation Analysis (36)] |
| 10. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 274] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 11. | Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:264-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms - Review Article. Rofo. 2017;189:1055-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Fang L, Meng X, Luo W, Zhou XD. Treatment of primary hepatic carcinoma through ultrasound-guided microwave ablation. Niger J Clin Pract. 2019;22:1408-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lachenmayer A, Tinguely P, Maurer MH, Frehner L, Knöpfli M, Peterhans M, Weber S, Dufour JF, Candinas D, Banz V. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int. 2019;39:1975-1985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Song H, Ding H, Zhu C. CT-Guided Percutaneous Microwave Ablation of Sclerosing Hepatic Carcinoma. Can J Gastroenterol Hepatol. 2020;2020:8881978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Cui R, Yu J, Kuang M, Duan F, Liang P. Microwave ablation versus other interventions for hepatocellular carcinoma: A systematic review and meta-analysis. J Cancer Res Ther. 2020;16:379-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Melikian R, Minocha J. Septic Shock and Death after Microwave Ablation of Hepatocellular Carcinoma in a Liver Transplant Patient with a Bilioenteric Anastomosis. Semin Intervent Radiol. 2019;36:137-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 19. | Wang T, Zhang XY, Lu X, Zhai B. Laparoscopic Microwave Ablation of Hepatocellular Carcinoma at Liver Surface: Technique Effectiveness and Long-Term Outcomes. Technol Cancer Res Treat. 2019;18:1533033818824338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Makovich Z, Logemann J, Chen L, Mhaskar R, Choi J, Parikh N, El-Haddad G, Kis B. Liver tumor ablation in difficult locations: Microwave ablation of perivascular and subdiaphragmatic hepatocellular carcinoma. Clin Imaging. 2021;71:170-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Schaible J, Lürken L, Wiggermann P, Verloh N, Einspieler I, Zeman F, Schreyer AG, Bale R, Stroszczynski C, Beyer L. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: comparison of stereotactic and conventional manual guidance. Sci Rep. 2020;10:18835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 23. | Ma B, Liu X, Yu Z. The effect of high intensity focused ultrasound on the treatment of liver cancer and patients' immunity. Cancer Biomark. 2019;24:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Yang G, Xiong Y, Sun J, Wang G, Li W, Tang T, Li J. The efficacy of microwave ablation versus liver resection in the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. Int J Surg. 2020;77:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Glassberg MB, Ghosh S, Clymer JW, Wright GWJ, Ferko N, Amaral JF. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Zhang YX, Zhang XH, Yu XL, Han ZY, Yu J, Liu FY, Cheng ZG, Liang P. Prognosis of microwave ablation for hepatocellular carcinoma: does age make a difference? Int J Hyperthermia. 2020;37:688-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Liu C, He J, Li T, Hong D, Su H, Shao H. Evaluation of the efficacy and postoperative outcomes of hydrodissection-assisted microwave ablation for subcapsular hepatocellular carcinoma and colorectal liver metastases. Abdom Radiol (NY). 2021;46:2161-2172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, Zhou X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Dumolard L, Ghelfi J, Roth G, Decaens T, Macek Jilkova Z. Percutaneous Ablation-Induced Immunomodulation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Darweesh SK, Gad AA. Percutaneous microwave ablation for HCV-related hepatocellular carcinoma: Efficacy, safety, and survival. Turk J Gastroenterol. 2019;30:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Meloni MF, Chiang J, Laeseke PF, Dietrich CF, Sannino A, Solbiati M, Nocerino E, Brace CL, Lee FT Jr. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia. 2017;33:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |