Copyright
©2014 Baishideng Publishing Group Inc.
World J Methodol. Dec 26, 2014; 4(4): 219-231
Published online Dec 26, 2014. doi: 10.5662/wjm.v4.i4.219
Published online Dec 26, 2014. doi: 10.5662/wjm.v4.i4.219
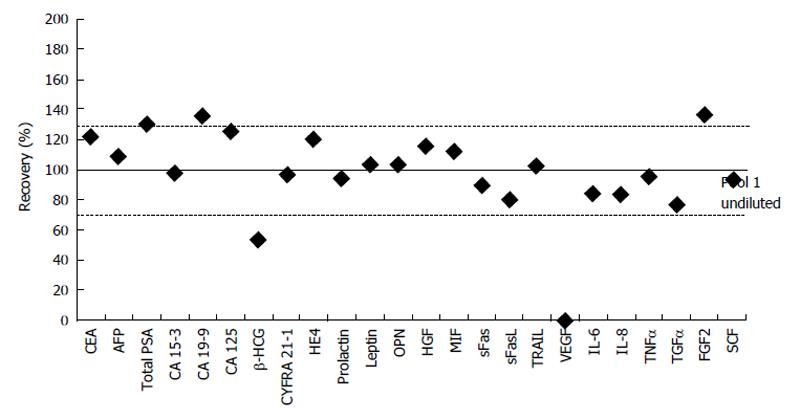
Figure 1 Dilution recovery.
Recoveries of a 50% dilution of serum pool 1 for all 24 biomarkers. The values are observed concentration-based and the corresponding measured undiluted pool 1 values were taken as reference. Horizontal lines depict the acceptable range of recovery. The dilution of VEGF fell below the measuring range. AFP: Alpha-fetoprotein; CA 125: Cancer antigen 125; CEA: Carcinoembryonic antigen; CV: Coefficient of variation; CYFRA 21-1: Cytokeratine 19-fragment; FGF2: Fibroblast growth factor-2; FI: Fluorescent intensity; HE4: Human epididymis protein 4; HGF: Hepatocyte growth factor; IL-6: Interleukin-6; MIF: Macrophage migration inhibitory factor; MR: Measuring range; Conc: Concentration; OPN: Osteopontin; QC 1: Quality control 1; SCF: Stem cell factor; sFas: Soluble Fas; sFasL: Soluble Fas-ligand; TGFα: Transforming growth factor-α; TNFα: Tumor necrosis factor-α; total PSA: Total prostate-specific antigen; TRAIL: Tumor necrosis factor related apoptosis-inducing ligand; VEGF: Vascular endothelial growth factor; β-HCG: β-human chorionic gonadotropin.
- Citation: Hermann N, Dreßen K, Schildberg FA, Jakobs C, Holdenrieder S. Methodical and pre-analytical characteristics of a multiplex cancer biomarker immunoassay. World J Methodol 2014; 4(4): 219-231
- URL: https://www.wjgnet.com/2222-0682/full/v4/i4/219.htm
- DOI: https://dx.doi.org/10.5662/wjm.v4.i4.219