Copyright
©2013 Baishideng Publishing Group Co.
World J Methodol. Dec 26, 2013; 3(4): 45-64
Published online Dec 26, 2013. doi: 10.5662/wjm.v3.i4.45
Published online Dec 26, 2013. doi: 10.5662/wjm.v3.i4.45
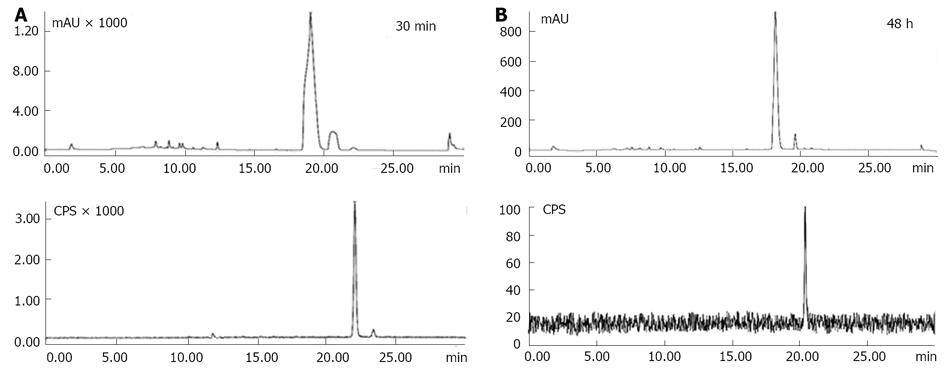
Figure 5 High performance liquid chromatographic analysis of the non-purified iodine-123 labeled hypericin with Ultraviolet (254 nm) and radiometric detection.
A: Ultraviolet-chromatogram of the starting reagent hypericin (Hyp) with a retention time of 19.18 min (upper part) and radiochromatogram (lower part) of 123I-Hyp eluting at 22.13 min with a mean radiochemical yield of 95.4%; B: High performance liquid chromatographic analysis of the non-purified 123I-Hyp at 48 h after labeling. A single narrow peak coming out at 21.90 min (lower part) suggests the in vitro stability of 123I-Hyp over time. CPS: Count per second.
- Citation: Cona MM, Witte P, Verbruggen A, Ni Y. An overview of translational (radio)pharmaceutical research related to certain oncological and non-oncological applications. World J Methodol 2013; 3(4): 45-64
- URL: https://www.wjgnet.com/2222-0682/full/v3/i4/45.htm
- DOI: https://dx.doi.org/10.5662/wjm.v3.i4.45