Copyright
©The Author(s) 2015.
World J Transl Med. Dec 12, 2015; 4(3): 69-77
Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.69
Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.69
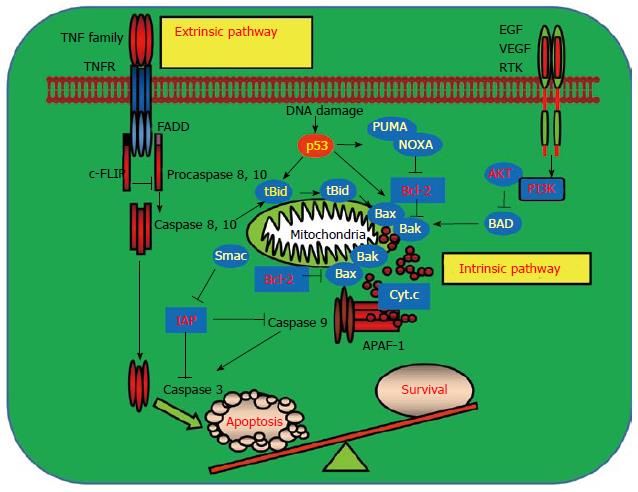
Figure 1 Signaling pathways mediating programmed cell death and cell survival.
Apoptosis can be induced by extra- and intra-cellular stimuli. Activation of the cell surface receptors such as tumor necrosis factor receptor (TNFR) upon binding to their ligands leads to activation of the receptor-mediated extrinsic apoptotic pathway. Activation of death receptors triggers the cleavage of pro-caspases 8 and 10 to their active forms by recruiting adaptor protein, Fas associated via death domain (FADD) to form a death inducing signaling complex (DISC). The activated caspases 8 and 10 then activate the downstream effector caspase 9 and result in apoptosis. DISC formation is negatively regulated by cellular Fas-associated death domain-like interleulin-1β converting enzyme inhibitory protein (c-FLIP) which inhibits apoptosis. Intracellular stresses such as DNA damage, oncogene activation and virus infection activate the intrinsic apoptotic pathway which usually requires p53 function. p53 triggers the intrinsic apoptotic pathway by upregulating its downstream proapoptotic target genes including the p53 up-regulated modulator of apoptosis (PUMA), phorbol-12-myristate-13-acetate-induced protein 1 (NOXA), Bcl-2 associated X protein (Bax) and Bcl-2 antagonist killer1 (Bak), which in turn lead to the release of cytochrome c from the mitochondrion. In the cytosol, the released cytochrome c binds to apoptotic protease activating factor 1 (APAF-1) and caspase 9 to form the apoptosomes. Activated caspase 9 then cleaves and activates the effector caspases 3, 6, and 7 to execute apoptosis. The mitochondrial protein Smac augments apoptosis by interacting and inhibiting inhibitor of apoptosis proteins (IAPs) which block the activation of caspase 9. Akt, an important downstream target in response to the activation of receptor tyrosine kinases, inhibits apoptosis by phosphorylating and inhibiting the proapoptotic Bcl-2 associated antagonist of cell death (BAD) which prevents the activation of Bax and Bak. Bid crosslinks the extrinsic and intrinsic pathways in response to extrinsic stimuli. Activation of caspases 8 and 10 causes the cleavage of BH3 interacting domain death agonist (BID) and the truncated BID is then translocated to the mitochondrion to engage the activation of the intrinsic pathway by interacting with Bax and Bak. EGF: Epidermal growth factor; VEGF: Vascular endothelial growth factor; RTK: Receptor tyrosine kinase.
- Citation: Liu XC, Gao JM, Liu S, Liu L, Wang JR, Qu XJ, Cai B, Wang SL. Targeting apoptosis is the major battle field for killing cancers. World J Transl Med 2015; 4(3): 69-77
- URL: https://www.wjgnet.com/2220-6132/full/v4/i3/69.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i3.69