Peer-review started: June 9, 2016
First decision: July 5, 2016
Revised: December 23, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 6, 2017
Processing time: 273 Days and 15.1 Hours
To determine acute kidney in jury (AKI) incidence and potential risk factors of AKI in children undergoing spinal instrumentation surgery.
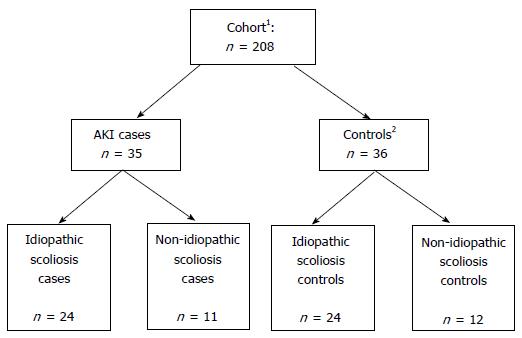
AKI incidence in children undergoing spinal instrumentation surgery at British Columbia Children’s Hospital between January 2006 and December 2008 was determined by the Acute Kidney Injury Networ classification using serum creatinine and urine output criteria. During this specific time period, all patients following spinal surgery were monitored in the pediatric intensive care unit and had an indwelling Foley catheter permitting hourly urine output recording. Cases of AKI were identified from our database. From the remaining cohort, we selected group-matched controls that did not satisfy criteria for AKI. The controls were matched for sex, age and underlying diagnosis (idiopathic vs non-idiopathic scoliosis).
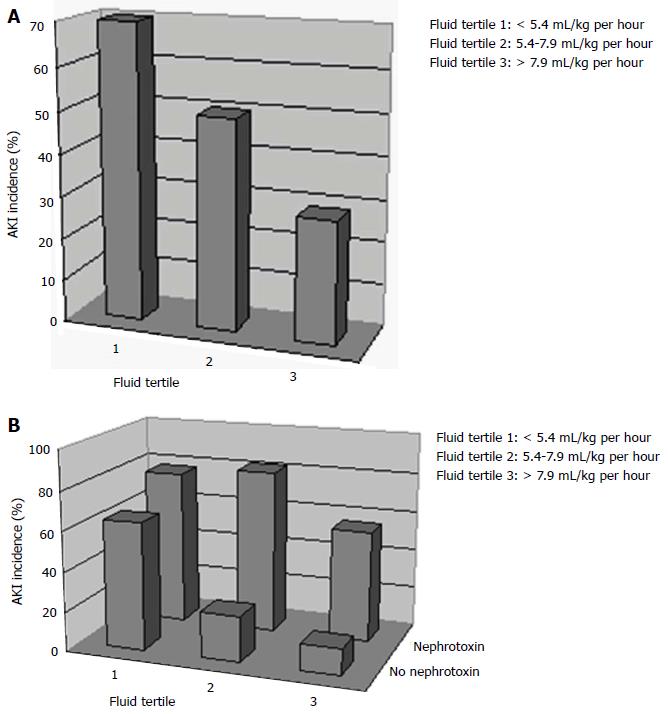
Thirty five of 208 patients met criteria for AKI with an incidence of 17% (95%CI: 12%-23%). Of all children who developed AKI, 17 (49%) developed mild AKI (AKI Stage 1), 17 (49%) developed moderate AKI (Stage 2) and 1 patient (3%) met criteria for severe AKI (Stage 3). An inverse relationship was observed with AKI incidence and the amount of fluids received intra-operatively. An inverse relationship was observed with AKI incidence and the amount of fluids received intra-operatively classified by fluid tertiles: 70% incidence in those that received the least amount of fluids vs 29% that received the most fluids (> 7.9, P = 0.02). Patients who developed AKI were more frequently exposed to nephrotoxins (non steroidal anti inflammatory drugs or aminoglycosides) than control patients during their peri-operative course (60% vs 22%, P < 0.001).
We observed a high incidence of AKI following spinal instrumentation surgery in children that is potentially related to the frequent use of nephrotoxins and the amount of fluid administered peri-operatively.
Core tip: We are the first to report a high incidence of acute kidney injury (AKI) of 17% in children undergoing orthopaedic spinal instrumentation surgery utilizing the Acute Kidney Injury Network definition. A relationship was observed between the development of AKI and the use of nephrotoxins including non-steroidal anti-inflammatory drugs and lower amounts of intravenous fluid administered peri-operatively. These results suggest that there are modifiable AKI risk factors with the potential of reducing AKI incidence in this understudied population. Further prospective studies with the use of novel AKI biomarkers are needed to validate our novel results.
- Citation: Jöbsis JJ, Alabbas A, Milner R, Reilly C, Mulpuri K, Mammen C. Acute kidney injury following spinal instrumentation surgery in children. World J Nephrol 2017; 6(2): 79-85
- URL: https://www.wjgnet.com/2220-6124/full/v6/i2/79.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i2.79
Over the past decade, there has been a major epidemiological shift in paediatric acute kidney injury (AKI) etiology. Previously, AKI was most often caused by primary renal diseases. Currently, the majority of AKI cases are related to secondary insults including sepsis and nephrotoxins. A high incidence of AKI has been reported in various paediatric populations including 5% of all paediatrics hospitalizations, 10%-17.9% of paediatric intensive care unit (PICU) admissions, 82% of the most severely-ill children admitted to the PICU and up to 62% of infants following cardiac surgery[1-7]. Several studies have also revealed an increased morbidity and mortality associated with pediatric AKI in critically-ill children and an increased risk of chronic kidney disease in survivors of AKI[1-8].
Outside of cardiac surgery patients, the epidemiology of AKI has not been well studied in other paediatric surgical populations including those undergoing major orthopaedic surgeries. The aim of this retrospective study was to define the incidence of AKI in children following spinal instrumentation surgery and to identify potential modifiable risk factors that may direct potential change in future practice.
We performed a nested case-control study of children undergoing orthopaedic spinal instrumentation surgery for scoliosis at BC Children’s Hospital (Vancouver, Canada). From a local database, we identified a cohort of 208 patients who had undergone spinal instrumentation surgery at our centre between January 2006 and December 2008. During this specific time period, all patients following spinal surgery were monitored in the PICU for at least 24 h post-operatively. All patients had an indwelling Foley catheter during their stay in the PICU with hourly urine output recording. Cases of AKI were identified from our database using the following criteria as per the Acute Kidney Injury Network (AKIN) definition: A 50% rise in serum creatinine (SCr) and/or urine output (U/O) < 0.5 mL/kg per hour for at least 6 h. AKIN staging criteria (stages 1-3), are shown in Table 1. From the remaining cohort, we selected group-matched controls that did not satisfy criteria for AKI. The controls were matched for sex, age and underlying diagnosis (idiopathic vs non-idiopathic scoliosis) (Figure 1). Abstracted data from our database included demographics (age at surgery, gender), pre-operative data (weight and height, hemoglobin, SCr, serum sodium, baseline blood pressure), intra-operative data (duration of surgery, intravenous fluid intake, urine output, blood pressure monitoring, use of nephrotoxins including non-steroidal anti-inflammatory drugs (NSAIDs) and aminoglycosides as well as post-operative data from the PICU and hospital admissions (serial SCr measurements, serum sodium, hourly urine output until Foley catheter removed, fluid intake, nephrotoxin use, ICU/hospital length of stay, and mortality). Data were supplemented, if required, with further chart review. Study data were collected and managed using REDCap electronic data capture tools hosted at BC Children’s Hospital.
| Acute Kidney Injury Network criteria | Stage 1 | Stage 2 | Stage 3 |
| Serum creatinine | 1.5-1.9 times baseline or ≥ 0.3 mg/dL (≥ 26.5 μmol/L) increase | 2.0-2.9 times baseline | 3.0 times baseline or ≥ 4 mg/dL (353.6 μmol/L) increase or Need for RRT |
| Urine output | < 0.5 mL/kg per hour for ≥ 6-12 h | < 0.5 mL/kg per hour for ≥ 12 h | < 0.3 mL/kg per hour for ≥ 24 h or Anuria ≥ 12 h |
All statistical analyses were performed with SPSS software (v 18.0; IBM SPSS Software for Predictive Analytics). Continuous variables following a normal distribution were expressed as mean and standard deviation (SD). Variables following a non-normal distribution were expressed as median and interquartile range (IQR). Categorical variables were expressed as proportions. Two-sided t tests were used to compare means. χ2 test was used to compare proportions. Mann-Whitney U test and Kruskall-Wallis tests were used to compare medians as appropriate. In all analyses, a P value < 0.05 was considered statistically significant. We divided patients into tertiles based on their intra-operative fluid administration in milliliter/kilogram per hour (corrected for patient size and duration of surgery) to explore the relationship between intra-operative fluid practices and AKI (comparison of AKI incidence between fluid tertiles). We also identified peri-operative (surgical or ICU) nephrotoxin exposure in each of the fluid tertiles to determine the relationship of fluid administration and nephrotoxin exposure to the development of AKI.
Thirty five of the 208 patients met criteria for AKI with an incidence of 17% (95%CI: 12%-23%). A total of 36 patients were selected as controls. Table 2 lists the patient baseline pre-operative characteristics. No clinically significant differences were noted between AKI and non-AKI patients including demographics, pre-operative renal function, and haemoglobin levels. Forty-eight (68%) of patients had surgery for “idiopathic” scoliosis. Twenty three (32%) had surgery for “non-idiopathic” scoliosis with primary diagnoses including Cerebral Palsy (11), Spondylolisthesis (4), Spina Bifida (2), Arnold-Chiari malformation (3) and miscellaneous (3). No patient had an elevated serum creatinine for age before surgery to suggest pre-operative AKI. However, patients with non-idiopathic scoliosis had a significantly lower baseline serum creatinine value of 38 μmol/L (SD 14.8) compared to 54 μmol/L (SD 10.9) in patients with idiopathic scoliosis (P = 0.05).
| Variables | AKI (n = 35) | Non-AKI (n = 36) | Total (n = 71) |
| Age (yr) | 15.4 ± 1.75 | 14.4 ± 1.95 | 14.9 ± 1.90 |
| Sex (male) | 9 (26%) | 6 (17%) | 15 (21%) |
| Idiopathic scoliosis | 24 (69%) | 24 (67%) | 48 (68%) |
| Weight (kg) | 56.5 ± 16.0 | 45.2 ± 11.9 | 50.7 ± 15.1 |
| Height (cm) | 161 ± 9.8 | 155 ± 10.6 | 158 ± 10.6 |
| BSA (kg/m2) | 1.58 ± 0.27 | 1.38 ± 0.23 | 1.48 ± 0.27 |
| Pre-op syst BP (mmHg) | 116 ± 13.4 | 113 ± 9.8 | 114 ± 11.7 |
| Pre-op diast BP (mmHg) | 71 ± 11.1 | 71 ± 8.9 | 71 ± 9.9 |
| Baseline creatinine (μmol/L) | 49.9 ± 15.2 | 47.4 ± 13.1 | 48.7 ± 14.1 |
| Baseline Hb (g/L) | 138 ± 11.7 | 137 ± 13.1 | 137 ± 12.4 |
Of all children who developed AKI, 17 (49%) developed mild AKI (AKIN Stage 1), 17 (49%) developed moderate AKI (AKIN stage 2) and 1 patient (3%) met criteria for severe AKI (AKIN stage 3). All patients met criteria for AKI based on urine output while only 2 patients met criteria for both serum creatinine and urine output change. Patients who met criteria for AKIN stages 1 and 2 did so after a mean of 9.1 (SD 4.2) and 13.8 (SD 3.4) h following admission to PICU respectively. The single patient with severe AKI based on urine output developed oliguria 24 h after PICU admission. The mean maximum serum creatinine rise (% from baseline) was higher in patients with AKI (0%, SD ± 22%) compared to patients without AKI (-7%, SD ± 12%), but did not reach statistical significance (P = 0.09).
Table 3 compares several intra-operative and post-operative (PICU admission) variables between AKI and non-AKI patients. Overall, mean duration of surgery was 8.4 h (SD 2.3 h). Duration of surgery, decreases in blood pressure during surgery, percentage requiring blood transfusions, and decreases in haemoglobin levels were similar in AKI and non-AKI patients (all P > 0.05). There was wide practice variation in intra-operative fluid volume administration between patients. All intravenous fluid administered was isotonic. The mean recorded urine output during surgery was 2.1 (SD 1.4) mL/kg per hour and was not statistically different between AKI and non-AKI patients [1.9 mL/kg per hour (SD 1.1) vs 2.4 mL/kg per hour (SD 1.5) respectively (P = 0.17)]. We classified all children into tertiles according to intra-operative fluids received, corrected for patient weight and surgery duration: < 5.4 mL/kg per hour, 5.4-7.9 mL/kg per hour, and > 7.9 mL/kg per hour. An inverse relationship was observed with the incidence of AKI and the amount of fluids received intra-operatively: 70%, 50%, 29% AKI incidence in the 1st, 2nd and 3rd fluid tertiles respectively (P = 0.02) (Figure 2A). The lowest recorded mean intra-operative serum sodium was 138 mmol/L (SD 2.4) while 4 patients (2%) developed hyponatremia during surgery (serum sodium < 135 mmol/L). During surgery, 14 out of 71 patients (19.7%) were exposed to a nephrotoxic medication (either aminoglycosides or NSAIDs). The majority of these patients (78.6%) received a dose of intravenous Ketorolac (NSAID) while 21.4% were exposed to intravenous Gentamycin (aminoglycoside). Overall, there was no difference in intra-operative nephrotoxin exposure between AKI and control groups (23% vs 17%) (P = 0.5).
| AKI (n = 35) | Non-AKI (n = 36) | P value | |
| Intra-operative course | |||
| Duration of surgery (h) | 8.5 ± 2.4 | 8.2 ± 2.1 | 0.54 |
| Cumulative intra-operative fluid administration (mL) | 2555 ± 973 | 2732 ± 1264 | 0.51 |
| Cumulative intra-operative fluid administration (mL/kg per hour ) | 6.2 ± 2.9 | 7.5 ± 3.3 | 0.08 |
| Intra-operative urine output (mL/kg per hour ) | 1.9 ± 1.1 | 2.4 ± 1.5 | 0.17 |
| Intra-operative fluid balance (L) | 1.2 ± 1.0 | 1.3 ± 1.1 | 0.70 |
| Max BP decline during surgery | |||
| Systolic (% from baseline) | 27 ± 12 | 33 ± 14 | 0.36 |
| Diastolic (% from baseline) | 30 ± 9 | 34 ± 13 | 0.93 |
| Patients requiring blood transfusion [n (%)] | 7 (20%) | 12 (33%) | 0.21 |
| Nephrotoxin exposure [n (%)] | 8 (23%) | 6 (17%) | 0.51 |
| Post-operative course | |||
| Duration of PICU stay (d) | 1 (IQR 0, range 17) | 1 (IQR 0, range 6) | 0.77 |
| Cumulative PICU fluid administration (mL) | 2069 (IQR 886) | 1918 (IQR 1048) | 0.15 |
| Cumulative PICU fluid administration (mL/kg per hour) | 2.1 ± 0.8 | 2.3 ± 0.7 | 0.31 |
| PICU urine output (mL/kg per hour) | 0.7 ± 0.4 | 1.1 ± 0.4 | < 0.001 |
| PICU fluid balance (L) | 1.3 ± 1.0 | 0.8 ± 0.8 | 0.02 |
| Max Hb drop (% from baseline) | 26 ± 9 | 26 ± 10 | 0.94 |
| Nephrotoxin exposure in PICU | 18 (51%) | 4 (11%) | < 0.001 |
A detailed summary of post-operative variables in AKI and non-AKI patients is shown in Table 2. Mean urine output was reduced at 0.9 (SD 0.4) mL/kg per hour during the PICU admission. Patients who developed AKI post-operatively had significantly lower urine output (0.7 mL/kg per hour vs 1.1 mL/kg per hour) and a higher cumulative fluid balance (positive 1.3 L vs positive 0.8 L) compared to control patients during their PICU admission (both P < 0.05). The lowest recorded mean post-operative serum sodium was 137 mmol/L (SD 2.8). Overall, 10 patients (14%) developed hyponatremia either during surgery or post-operatively. In patients who developed hyponatremia, the mean value of lowest sodium recorded was 133 mmol/L (SD 1.5). Patients who developed AKI were exposed to nephrotoxins (either aminoglycosides or NSAIDs) more frequently than control patients during their PICU admission (51% vs 11%, P < 0.001). Ninety-one percent of the patients exposed to NSAIDs received intravenous Ketorolac while the remainder received oral Ibuprofen. All patients with aminoglycoside exposure received intravenous Gentamycin. Overall, 21/35 (60%) patients who developed AKI were exposed to a nephrotoxic medication either during surgery or ICU admission compared to 8/36 (22%) of controls (P = 0.001). Figure 2B shows the relationship of intra-operative fluid administration and peri-operative nephrotoxin exposure on the development of AKI. The highest incidence of AKI was seen in patients exposed to less amounts of fluids intra-operatively (1st and 2nd fluid tertiles) while also being exposed to a nephrotoxin (75% and 80% AKI incidence respectively) while the lowest AKI incidence was observed in patients exposed to the highest amounts of fluids (3rd fluid tertile) with no exposure to a nephrotoxin (10% AKI incidence) (P = 0.04). Thirteen patients (18%) required mechanical ventilation in PICU with a mean duration of 1.8 (SD 1.2) d. Of those 13 children, 7 (20%) were in the AKI group vs 6 (17%) in the non-AKI group. Children with AKI were ventilated longer (mean 2.2 d vs 1.4 d in the control group; however this difference was not statistically significant (P = 0.7).
There were no cases of mortality seen in any of the patients following surgery. Median hospital length of stay in all patients was 6 d (IQR 3, range 4-49 d). Median ICU length of stay in all patients was 1 d (IQR 0, range 0-18 d). ICU length of stay was similar in AKI (median 1 d, IQR 0 d, range 17 d) and non-AKI patients (median 1 d, IQR 0 d, range 6 d) (P = 0.95). Similarly, hospital length of stay was similar in patients with AKI (median 6 d, IQR 3 d, range 25) and without AKI (median 6 d, IQR 2 d, range 45 d, P = 0.77). However, the single patient with severe AKI (AKIN stage 3) had a more prolonged PICU and hospital length of stay at 4 and 12 d respectively.
We report a high estimated AKI incidence of 17% in children undergoing orthopaedic spinal instrumentation surgery utilizing a standardized AKI definition. A number of recent adult studies have reported AKI incidences from 3.9%-16% following major orthopaedic surgeries[9-11]. There have been no prior epidemiological AKI studies in any paediatric orthopaedic populations.
In our cohort, no patient had pre-existing kidney dysfunction prior to surgery, which suggests that our high incidence of AKI may be primarily related to peri-operative risk factors. We identified 2 potential risk factors that may be related to the development of AKI in this population: (1) lower intravenous fluid exposure in the intra-operative period; and (2) high peri-operative nephrotoxin exposure with 60% of the AKI population being exposed to NSAIDs or aminoglycosides. The exact interaction between peri-operative fluid and nephrotoxin exposure towards the development of AKI cannot be determined in our retrospective analysis, but our results suggest that patients who are exposed to the highest amounts of fluids intra-operatively without nephrotoxin exposure may be more protected against AKI. Interestingly, a recent adult orthopaedic study showed that peri-operative dehydration, administration of NSAIDs, and use of nephrotoxic antibiotics were all independently associated with the development of post-operative kidney dysfunction[11]. According to a large epidemiological study analyzing the most common AKI etiologies in children, AKI related to nephrotoxin exposure accounts for up to 16% of in-hospital paediatric AKI[12]. Outside of fluid intake and use of nephrotoxins, there may be other complex intra-operative factors related to AKI that we were not able to study based on the retrospective nature of our study. For example, a recent local observational study involving 30 children undergoing scoliosis surgery found that a significantly reduced cardiac output (18.5% reduction)was associated with the prolonged prone positioning required for these surgeries[13]. Reduced cardiac output may lead to a state of poor renal perfusion during these lengthy procedures, which may put these children at a higher risk of AKI compared to other general surgical populations.
Even though the rise of creatinine trended higher in AKI patients compared to controls, it is interesting to note that the majority of AKI patients in our cohort were “oliguric” without significant rises in serum creatinine that often did not meet the creatinine criteria of the AKIN definition (at least 50% rise in creatinine). This may be partly related to a reduction of serum creatinine concentration associated with hemo-dilution effects and relative fluid overload as the majority of our patients demonstrated a cumulative positive fluid balance intra-operatively and in the ICU post-operatively. In addition, approximately 1/3 of our surgical cohort had an underlying condition including spina bifida and cerebral palsy (non-idiopathic scoliosis population). The significantly lower baseline serum creatinine values observed in our non-idiopathic scoliosis patients implies that a significantly low muscle mass state may limit an appreciable rise in serum creatinine during an episode of AKI. This is also a population where the use of recently validated AKI biomarkers representing early tubular damage [e.g., neutrophil gelatinase associated lipocalin (NGAL), interleukin-18 (IL-18), and kidney-injury molecule (KIM-1)] may be warranted in future orthopaedic AKI studies due to the potential issues of serum creatinine in this highly prevalent group of patients[14-16]. Lastly, serum creatinine, was not monitored frequently past 24 h post-operatively in the majority of patients with the potential of higher rises in creatinine being missed in many patients.
Another potential reason for oliguria in this population is the syndrome of inappropriate anti-diuretic hormone (SIADH), which has been reported in adult and some paediatric spinal orthopedic literature[17-19]. The diagnosis of SIADH requires the presence of hyponatremia (serum sodium < 135 mmol/L). In our cohort, only 10 patients (14%) overall were recorded with hyponatremia either during surgery or post-operatively, and only 5 of the AKI patients (14%) had documented hyponatremia. Mean serum sodium levels were the same in AKI and non-AKI patients both intra (138) and post-operatively (137). Even though we were not able to completely exclude SIADH with serum ADH levels and urine osmolality values, it is unlikely that SIADH was the primary cause of oliguria based on several reasons. As shown in a paediatric study of SIADH in children following spinal fusion surgery, serum ADH levels peaked immediately post-op and declined by 6 h after surgery[20]. Our stage 1 AKI patients (n = 17) met criteria for oliguria (< 0.5 mL/kg per hour for at least 6 h) at a mean of 9.1 h after surgery and stage 2 AKI patients (n = 17) met criteria (< 0.5 cc/kg per hour for at least 12 h) at a mean of 13.8 h post-operatively, well after the peak of serum ADH levels was observed in prior studies[20]. In addition, the AKIN criteria for oliguria is strict (Stage 1: < 0.5 mL/kg per hour for at least 6 h) and is often more severe than what is observed in patients with SIADH. Therefore, SIADH as a primary reason for fulfilling AKIN urine output criteria is unlikely, but will need to be further explored in future prospective studies.
Unlike recent studies involving paediatric cardiac surgery patients, peri-operative AKI was not associated with morbidity (increased hospital/ICU length of stay) or mortality in children undergoing spinal instrumentation surgery. This needs to be studied prospectively in larger orthopaedic populations before any further conclusions are made due to the potential concerns of our study being under-powered with a relatively small sample size. Regardless, the relatively high incidence of AKI in our cohort cannot be ignored, especially in the context of several recent adult and paediatric publications revealing an increased risk of chronic kidney disease following a single episode of AKI including those of milder severity[3-8].
In conclusion, we observed a high incidence of AKI following spinal instrumentation surgery in children that is potentially related to the frequent use of nephrotoxins and the amount of fluid administered peri-operatively.
Outside of cardiac surgery patients, the epidemiology of acute kidney injury (AKI) has not been well studied in other paediatric surgical populations including those undergoing major orthopaedic surgeries.
Several studies have revealed an increased morbidity and mortality associated with pediatric AKI and an increased risk of chronic kidney disease in survivors of AKI.
The authors report a high incidence of AKI following spinal instrumentation surgery in children that is potentially related to the frequent use of nephrotoxins and the amount of fluid administered peri-operatively.
This study suggests that there are modifiable AKI risk factors with the potential of reducing AKI incidence in this understudied population.
Acute kidney injury network (AKIN)-criteria: A set of criteria based on urine output or rise in serum creatinine to define AKI, according to the AKIN.
This is an interesting study which is well done and with new findings. It will be interesting to get more data about renal function after 3-5 years to have an idea about the impact of AKI on the long term renal prognosis.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bhimma R, Friedman EA, Mahmoud KM, Olowu WA S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15:R146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 892] [Cited by in F6Publishing: 862] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165:522-527.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Viaud M, Llanas B, Harambat J. Renal outcome in long-term survivors from severe acute kidney injury in childhood. Pediatr Nephrol. 2012;27:151-152; author reply 153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Slack R, Hawkins KC, Gilhooley L, Addison GM, Lewis MA, Webb NJ. Long-term outcome of meningococcal sepsis-associated acute renal failure. Pediatr Crit Care Med. 2005;6:477-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1265] [Cited by in F6Publishing: 1494] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 9. | White SM, Rashid N, Chakladar A. An analysis of renal dysfunction in 1511 patients with fractured neck of femur: the implications for peri-operative analgesia. Anaesthesia. 2009;64:1061-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41:335-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005;45:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Brown ZE, Görges M, Cooke E, Malherbe S, Dumont GA, Ansermino JM. Changes in cardiac index and blood pressure on positioning children prone for scoliosis surgery. Anaesthesia. 2013;68:742-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: discovery, evaluation, and clinical application. Pediatr Nephrol. 2011;26:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:2151-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Brazel PW, McPhee IB. Inappropriate secretion of antidiuretic hormone in postoperative scoliosis patients: the role of fluid management. Spine (Phila Pa 1976). 1996;21:724-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Callewart CC, Minchew JT, Kanim LE, Tsai YC, Salehmoghaddam S, Dawson EG, Delamarter RB. Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion in adult spinal surgery. Spine (Phila Pa 1976). 1994;19:1674-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Mason RJ, Betz RR, Orlowski JP, Bell GR. The syndrome of inappropriate antidiuretic hormone secretion and its effect on blood indices following spinal fusion. Spine (Phila Pa 1976). 1989;14:722-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Lieh-Lai MW, Stanitski DF, Sarnaik AP, Uy HG, Rossi NF, Simpson PM, Stanitski CL. Syndrome of inappropriate antidiuretic hormone secretion in children following spinal fusion. Crit Care Med. 1999;27:622-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |