Published online Nov 6, 2015. doi: 10.5527/wjn.v4.i5.511
Peer-review started: April 15, 2015
First decision: May 13, 2015
Revised: August 13, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: November 6, 2015
Processing time: 211 Days and 12.5 Hours
Acute kidney injury (AKI) is a common complication in patients with end-stage liver disease and advanced cirrhosis regardless of the underlying cause. Hepatorenal syndrome (HRS), a functional form of kidney failure, is one of the many possible causes of AKI. HRS is potentially reversible but involves highly complex pathogenetic mechanisms and equally complex clinical and therapeutic management. Once HRS has developed, it has a very poor prognosis. This review focuses on the diagnostic approach to HRS and discusses the therapeutic protocols currently adopted in clinical practice.
Core tip: Hepatorenal syndrome is a functional and potentially reversible form of kidney failure. The pathophysiological bases of this disease are complex and not fully understood. The aim of this review is to focus the current diagnostic approach and the updated therapeutic protocols adopted in clinical practice.
- Citation: Baraldi O, Valentini C, Donati G, Comai G, Cuna V, Capelli I, Angelini ML, Moretti MI, Angeletti A, Piscaglia F, Manna GL. Hepatorenal syndrome: Update on diagnosis and treatment. World J Nephrol 2015; 4(5): 511-520
- URL: https://www.wjgnet.com/2220-6124/full/v4/i5/511.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i5.511
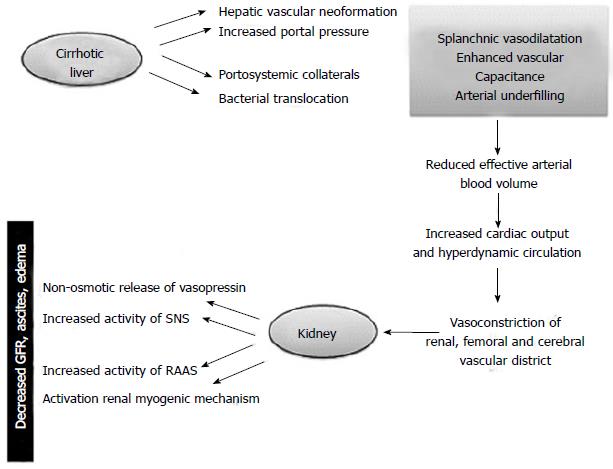
Hepatorenal syndrome (HRS) can be considered the final stage of a pathophysiological condition characterized by decreased renal blood flow resulting from deteriorating liver function in patients with cirrhosis and ascites[1-5].
Hemodynamic changes associated with endothelial shear stress occur before the onset of ascites and are sustained by an increase in pro-angiogenic factors like the vascular endothelial growth factor and platelet-derived growth factor and vasodilators (carbon monoxide, endocannabinoids and nitric oxide) able to promote the formation of hepatic, splanchnic and portosystemic collateral vessels[6-11] (Figure 1).
The ensuing hemodynamic instability may give rise to many clinical events that further interfere with the compensatory mechanisms. These include the onset of spontaneous bacterial peritonitis, gastrointestinal bleeding and post-paracentesis circulatory dysfunction[12].
The renal impairment is worsened by a progressive cardiac dysfunction known as cirrhotic cardiomyopathy. The latter is characterized by diastolic impairment with septal ventricular hypertrophy, blunted ventricular response to stress, systolic and diastolic dysfunction, and electrophysiological abnormalities (prolongation of QT interval)[7]. Systolic dysfunction is due to impairment of both β-adrenergic receptor and increasing in endogenous cannabinoids and cardiosuppressants such as nitric oxide and inflammatory cytokines and myocyte apoptosis. Furthermore it is possible that several intracellular signaling pathways are involved.
On the other hand the activation of renin-angiotensin system and salt retention play a role in diastolic disfunction. Recent studies have stated myocardial dysfunction in cirrhosis as a contributing, or even a precipitant factor, of HRS[13,14] .
According to Fede et al[15], approximately 20% of cirrhotic patients with diuretic-resistant ascites potentially develop HRS, while a prospective study by Ginès et al[4] on 229 patients with cirrhosis found an 18% incidence of HRS at one year, rising to 39% at five years after initial diagnosis.
HRS may also arise in patients with acute liver failure as shown in Akriviadis et al[16]: They considered 101 patients with alcoholic hepatitis of whom 28 developed HRS after a four-week follow-up. Planas et al[17], in a study enrolling 263 cirrhotic patients with a follow-up of 41 ± 3 mo after the onset of ascites, found prevalence rates of 2.6% and 5% for HRS types I and II respectively, with a cumulative probability of 11.4% at five years. The prevalence of HRS increases with liver disease progression, Wong et al[18] reporting a rate of 48% in patients on the waiting list for liver transplant.
Despite discrepancies in literature data, the prevalence of HRS has dropped in recent years, probably as a result of a better understanding of its pathophysiology and improved clinical management[19]. Nonetheless the long-term survival of HRS patients remains poor and the only effective treatment for this condition is liver transplantation.
The diagnostic criteria for HRS were initially defined by the International Ascites Club (IAC) in 1994[20-22]. Since then, advances in our understanding of HRS pathogenesis and the introduction of new therapies led to repeated revisions of the criteria. The latest version of 2007 excludes the use of creatinine clearance (due to its poor correlation with kidney function in patients with cirrhosis), and has eliminated minor criteria (sodium excretion fraction, urinary output) deemed less sensitive and specific. Concomitant bacterial infection does not rule out a diagnosis of HRS but it is crucial to identify the absence of septic shock[1] (Table 1).
| Cirrhosis with ascites |
| Serum Creatinine > 1.5 mg/dL |
| Absence of shock |
| No improvement of serum creatinine (decrease to a level of 1.5 mg/dL or less) after at least 2 d of diuretic withdraw and volume expansion with albumin (The recommended dose of albumin is 1 g/kg of body weight per day up to a maximum of 100 g/d) |
| No current o recent exposure to nephrotoxic drugs |
| Absence of parenchymal disease as indicated by proteinuria > 500 mg/d, microscopic hematuria (50 red blood cells per high power field) and abnormal renal ultrasonography |
Two forms of HRS, types I and II, have been described. They differ in severity and rate of progression and can be considered two separate clinico-pathological entities[23] (Table 2).
| HRS I | Doubling of serum creatinine in < 2 wk | A precipitating event is present in the most of case | No history of diuretic resistant ascites | 10% survival in 90 d without treatment |
| HRS II | Renal impairment gradually progressive | No precipitating events | Always ascites diuretic resistance | Median survival 6 mo |
Type I HRS is characterized by acute onset and rapidly progressing kidney failure with a doubling of serum creatinine to > 2.5 mg/dL (corresponding to a 50% reduction in the creatinine clearance rate) in less than 2 wk, usually associated with multiorgan damage. The prognosis is poor with only 10% of patients surviving longer than 90 d[4].
This type of HRS can develop spontaneously but more often tends to follow a precipitating event, mostly spontaneous bacterial peritonitis or other infections like pneumonia, urinary tract infections or cellulitis[24]. Other potential risk factors include viral, alcoholic, toxic or ischemic hepatitis (e.g., TIPS), gastrointestinal bleeding and surgical procedures (Table 3).
| Spontaneous bacterial peritonitis |
| Large volume paracentesis (> 5 L) with inadequate albumin substitution |
| NSAID and other nephrotoxic drugs, iv contrast |
| Bleeding from esophageal varices |
| Post TIPS syndrome |
| Diuretic treatment |
Type II HRS represents the final kidney response to hemodynamic impairments in cirrhosis. This type presents as a less severe and more gradual decline in renal function associated with refractory ascites. The increase in creatinine is gradual with mean values of 1.5-2.0 mg/dL. Type II HRS predisposes patients to the development of type I HRS after a precipitating event. The average survival rate is six to eight months after onset.
The differential diagnosis between the two types of HRS is based on the rate of progression and extent of renal impairment, whereas the pathophysiological differences have not yet been fully clarified.
A spontaneous recovery is rare in both cases unless there is a significant improvement in liver function.
The differential diagnosis between HRS, other causes of kidney disease and septic shock remain extremely difficult. Despite the widespread circulation of the IAC criteria, a serum creatinine cut-off of 1.5 mg/dL appears limited as it does not take into account its physiological fluctuations. In addition, creatinine values ≤ 1.5 mg/dL may overestimate the true reduction in GFR[25].
The AKI network (AKIN) has proposed a new definition of AKI for the diagnosis of HRS designed to implement the traditional IAC criteria for prompt recognition of kidney damage. AKI is defined as the abrupt loss of kidney function resulting in a 0.3 mg/dL increase in serum creatinine in 48 h or a 50% increase over the basal value. The aim is to apply the AKI criteria to decompensated cirrhotic patients for an early identification of kidney failure and thereby implementing prompt aggressive treatment[26].
Two recent prospective studies assessed the applicability of the AKI criteria in patients with cirrhosis. The study by Fagundes et al[27] on 375 patients and another by Piano et al[28] on 233 cirrhotic patients both divided the populations into two groups based on kidney function. The first group comprised patients with a serum creatinine increase ≥ 0.3 mg/dL but below the threshold of 1.5 mg/dL, whereas the second enrolled patients with creatinine > 1.5 mg/dL. In both cases renal decline and mortality rates were significantly higher in the group with serum creatinine > 1.5 mg/dL, with a lower probability of kidney disease regression. These results suggest that AKI with serum creatinine values < 1.5 mg/dL is a relatively benign and potentially reversible condition, whereas the progression of renal deterioration to a significant decrease in GFR (values > 1.5 mg/dL) carries a poor prognosis[27,28].
Nonetheless, a recent editorial by Arroyo et al[29] pointed to a lack of evidence demonstrating the real advantage of the IAC guidelines with respect to AKI criteria. The stratification of cirrhotic patients according to single organ damage (kidney, liver or brain) appears to simplify the complex changes occurring in patients with decompensated liver failure.
Mindikoglu et al[2] proposed a new classification associating GFR measurement and renal blood flow to stratify renal dysfunction, introducing the new concept of “pre-HRS”, i.e., patients with reduced renal blood flow but still normal or slightly reduced GFR. However, further studies are required to establish the clinical utility of this concept[30].
In all patients with acute renal failure and even more in patients with cirrhosis, serum creatinine may not reflect the reduction of kidney function with a significant difference between male and female. Because of that it was proposed using cystain C as alternative marker of renal function.
Seo et al[31] and Sharawey et al[32] showed that serum cystatin C level is a good marker for predicting HRS and survival in patients with cirrhotic ascites.
In the last 2 years the IAC organised a consensus development meeting in order to analyse the new definition of AKI in patients with cirrhosis and HRS: All the experts agreed on the removal of a fixed cut-off value of serum creatinine from the diagnostic criteria of HRS and they didn’t suggest to evaluate Cystain C determination[33] (Table 1).
As there are currently no specific tests to identify HRS, diagnosis rests on the exclusion of other causes of kidney failure. It is important to establish the etiology of kidney injury in order to institute the appropriate treatment.
The onset of AKI in patients with cirrhosis enters into the differential diagnosis with other forms of kidney injury: Pre-renal (45%), organic, including acute tubular necrosis and glomerulonephritis (32%), and less frequently obstructive nephropathy (< 1%)[34,35] (Table 4).
| Pre-renal | History of fluid loss, gastrointestinal bleeding, treatment with diuretics or non-steroidal anti-inflammatory drugs |
| Organic | Medical history, laboratory tests (cryoglobulinemia, complementemia, etc.) |
| Obstructive | Ultrasound imaging |
| Chronic kidney disease | Anemia, proteinuria, secondary hyperparathyroidism, ultrasound evidence of renal cortical thinning |
The parameters traditionally used to distinguish AKI from chronic kidney disease (CKD) (urinary sodium concentration, serum and urine osmolarity) are not applicable in patients with cirrhosis and ascites. Likewise, serum urea values are usually reduced in cirrhotic patients due to the impaired hepatic synthesis.
Belcher et al[36] proposed the use of urinary biomarkers of AKI to improve the diagnostic process: urinary levels of neutrophil gelatinase-associated lipocalin (NGAL), interleukin 18 (IL-18), kidney injury molecule-1 and liver fatty acid-binding protein are elevated in liver disease patients with kidney injury due to acute tubular necrosis.
Two recent trials studied patients admitted to hospital for cirrhosis-induced complications. They both demonstrated that raised urinary levels of NGAL may serve to distinguish functional kidney damage from acute tubular necrosis or necrosis arising in HRS[37,38].
Barreto et al[39] confirmed that urinary NGAL predicts clinical outcome, namely persistent kidney injury and mortality at three months in hospitalized patients with cirrhosis and bacterial infections. Although further clinical trials are required, NGAL appears to predict short-term mortality in cirrhotic patients.
Renal biopsy is not used for diagnostic purposes but can be entertained when a decline in renal function is associated with active urinary sediment or clinical status not corresponding to IAC criteria or unresponsive to therapy.
Despite improvements in the clinical management of HRS patients in the past twenty years, currently available treatments enhance patients’ short-term survival but offer little benefit in the longer term.
The current therapeutic armamentarium includes drugs with specific vasoconstrictive effects on the splanchnic circulation in addition to renal and liver replacement therapies which can be artificial or natural (liver transplantation). Liver transplant remains the only truly effective treatment but is limited by the high mortality rate in HRS patients and the shortage of available organs.
A recent literature review by Fabrizi et al[40] noted that pre-transplant kidney function is the most important predictor for patient survival after liver transplant. Pharmacological treatment and medical care serve as a “bridge” to transplant to improve the patient’s prognosis.
The cirrhotic patient with ascites must be closely monitored to prevent and treat precipitating factors[41-45] (Table 5).
| Avoid drugs that reduce renal perfusion or nephrotoxic substances |
| Minimize exposure to organ-iodated contrast agents |
| Intravenous albumin is recommended for volemic filling after large volume paracentesis (8 g of albumin for each liter of ascites removed) |
| Diuretic therapy should be suspended |
| Pentoxifylline as drug’s anti-TNFa activity |
| Antibiotic prophylaxis to prevent infections reducing intestinal bacterial translocation (norfloxacin 400 mg/d) |
| Intravenous albumin administered in association with ceftriaxone in SPB |
| Adrenal insufficiency should be identified and treated |
| Drug dosages must be adjusted according to renal function |
If multiorgan damage is present, some patients, especially those with type I HRS, may require a high level of care, and admission to an intensive care facility. In addition, a patient-tailored diet and physical rehabiligation program should be planned and each patient assessed for eligibility for liver transplantation to avoid aggressive treatment.
The aim of treatment must be to stabilize patients until liver transplantation and optimize their clinical condition for a successful transplant[6].
Medical management is targeted at the pathogenetic mechanisms underlying HRS. The ideal treatment is designed to improve liver function by exerting splanchnic vasoconstriction and renal vasodilation to reduce portal hypertension and raise systemic arterial pressure[34]. The specific drug approach is based on the use of vasoconstrictor agents (terlipressin, norepinephrine, midodrine) to correct circulatory changes.
As reported in a review by Davenport et al[41], intravenous administration of terlipressin and albumin is currently the treatment of choice for patients with type I and type II HRS, resulting in an overall reduction in short-term mortality rates.
The vasopressin synthetic analogue terlipressin is a V1 agonist of the receptors expressed on vascular smooth muscle cells in the splanchnic circulation. It is enzymatically transformed from the inactive to biologically active form (lysine-vasopressin) with a longer half-life than other vasopressin analogues, e.g., ornipressin. Terlipressin’s long half-life accounts for its initial administration as an intravenous bolus, now replaced by continuous infusion[46,47]. The vasoconstrictive effect of terlipressin corrects the circulatory dysfunction typical of end-stage liver disease, indirectly rebalancing intrarenal vasoconstriction and lowering levels of renin, noradrenaline and ultimately serum creatinine. As a result, the kidney regains control of its self-regulatory system. In addition, terlipressin has a major impact on the portal circulation reducing portal venous flow and porto-systemic pressure with a concomitant increase in hepatic arterial blood flow and an improvement in hepatocellular oxygenation.
Terlipressin can be administered as an intravenous bolus starting from a dose of 0.5 mg every 4-6 h or as a continuous infusion (2 mg/d). The dosage can be doubled after three days of treatment if there is no improvement in serum creatinine (i.e., a reduction of at least 25%)[12]. The total daily dose should not exceed 2 mg IV bolus every 4-6 h or 12 mg/d in continuous infusion[40]. Continuous infusion is associated with a better clinical response and fewer side-effects[48].
Terlipressin should be associated with albumin (at a dose of 1 g/kg per day on the first day, without exceeding 100 g/d, followed by 20-40 g/d). Albumin serves to expand the circulating plasma volume by raising the oncotic pressure. In addition, it has metabolic, immune and vasoconstrictor effects by binding to endotoxins, nitric oxide, bilirubin and fatty acids[49,50]. The terlipressin-albumin association improves renal function by 40%-60%[48], increasing the number of patients eligible for liver transplant thereby enhancing their outcome[51-53]. When serum creatinine values reach < 1.5 mg/dL, treatment is deemed complete[48]. The average recovery time is seven days up to a maximum of two weeks after which terlipressin should be suspended if there is no improvement in kidney function[54]. Even when there is a complete response, HRS recurrence is common (50% of cases) and treatment should be resumed.
Terlipressin has an acceptable side-effects profile. Side effects include abdominal pain with cramps and diarrhea until intestinal ischemia; cardiac tachiarrhythmias and chest pain can be observed, in generale ECG monitoring is recommended. Vasoconstriction induced by terlipressin may cause also cyanosis, livedo reticularis, necrosis of the skin and extremities[53]. Terlipressin could also associated with hyponatremia but without impairment of patients’ survival[55].
If patient shows side effects the dosage should be reduced or administration discontinued. Continuous infusion is safer and less burdened by side effects[52].
The incidence of ischemic events ranges from 5% to 30% even though many studies exclude patients at risk of cardiovascular ischemia. Fabrizi et al[56]’s literature meta-analysis of 243 patients compared the effects of terlipressin vs placebo on kidney function and survival in HRS patients. Their data confirm the regression of HRS in a significant number of treated patients but no effect on survival rates.
The association albumin and terlipressin showed an improvement of survival rates for positive effects of albumin on cardiac function, on the reduction of nitric oxid and on improving the responsiveness of arterial wall to vasoconstrictors. Other studies in patients treated with terlipressin and differents colloids didn’t showed the same positive response[52,53].
Terlipressin is not available in the United States and Canada so therapeutic protocols with other vasoconstrictor agents need to be considered in those countries.
The alpha-adrenergic receptor agonist norepinephrine has proved effective in the treatment of HRS. Continuous norepinephrine infusion (at a dose of 0.5-3 mg/h) must be associated with albumin administered as an IV bolus at least twice daily (1 g/kg up to a maximum of 100 g/d). The aim is to raise mean arterial pressure by 10 mmHg and urinary output > 200 mL every four hours. The maximum period of treatment must not exceed 2 wk[57,58].
A pilot study by Ghosh et al[59] compared terlipressin vs noradrenaline in 46 patients with type II HRS. Neither treatment proved superior to the other and the outcome was broadly the same in terms of HRS regression. Noradrenaline can be deemed as effective as terlipressin but its lower costs makes it an interesting option for the treatment of HRS.
Another alpha-adrenergic agent, midodrine, can be considered a good alternative to terlipressin and is the drug most commonly used in the United States. Midodrine is a prodrug metabolized by the liver into its active metabolite (desglymidodrine) and then excreted in the urine. When administered in association with octreotide (a somatostatin analogue and splanchnic vasodilator) it has a positive effect on renal function in HRS patients with 50% likelihood of disease reversal[49,60-62].
Midodrine can be administered orally (initial dose 7.5 mg every 8 h up to a maximum of 12.5 mg three times daily) or octreotide can be given by continuous infusion (50 mcg/h) or subcutaneously (from 100 to 200 mcg 12.5 mg three times daily). Albumin must be associated at the usual dose[6]. Midodrine dosage has a major effect on its effectiveness: Patients treated at the maximum dose have shown a complete response to therapy, whereas octreotide administered alone has no effect on kidney function[62].
The creation of a portosystemic shunt to treat refractory ascites can improve renal function in cirrhotic patients as it increases venous return of splanchnic blood to the right heart thereby raising the effective arterial blood volume and reducing hepatic sinusoidal pressure. Although literature reports on the use of transjugular intrahepatic portosystemic shunt (TIPS) in HRS patients are scant, Brensing et al[63] analyzed the trend of creatinine clearance in patients treated with TIPS, finding a twofold increase in clearance values from 9 to 27 mL/min two weeks after the procedure. Despite its side-effects (namely the high incidence of hepatic encephalopathy), TIPS can be used in the short term to gain potential benefits in patients awaiting liver transplant[64,65].
The indications for renal replacement therapy (RRT) in patients with HRS are the same as those for AKI patients without cirrhosis. HRS patients, particularly those with type I, may need to undergo dialysis because of metabolic acidosis or hyperkalemia due to water or sodium retention or less frequently uremic intoxication.
RRT is among the so-called bridging therapies designed to support patients awaiting liver transplant, but there is no evidence that dialysis improves the long-term survival of patients not eligible for transplantation[66].
By definition, patients with cirrhosis are at higher risk of bleeding and hemodynamic complications (hypotension, arrythmias) hampering the decision to initiate and manage dialysis treatment. Cirrhotic patients on RRT have a 2%-8% higher mortality rate than other patients[67].
Continuous renal replacement therapy (CRRT) is usually preferred to intermittent dialysis due to its greater hemodynamic stability ensuring fewer fluctuations in intracranial pressure. However, prospective studies show that the choice of RRT has no significant effect on survival rates in patients awaiting liver transplantation[68-71]. Anticoagulation of the extracorporeal circuit is needed to maintain the filter patency without increasing the risk of hemorrhage. Regional citrate anticoagulation emerged as possible alternative but no specific protocols are currently recommended for patients with liver diseases[72].
Peritoneal dialysis is an option to resolve ascites and correct other complications of cirrhosis without exposing patients to the complications of hemodialysis[73,74].
The precise timing and dose of RRT have yet to be established but some studies demonstrate that the early initiation and maintenance of a constantly negative fluid balance have a positive effect on survival rates[75].
More complex therapies known as liver support measures may be required to replace the liver’s detoxifying system. RRT removes water-soluble toxins whereas most of the molecules accumulated in the course of liver failure are linked to albumin and hence are not removed by conventional hemodialysis.
Liver support systems are designed to enhance and optimize these results, increasing the removal of water-soluble toxins and those linked to albumin.
To date these treatments have served as bridging therapies for patients awaiting liver transplantation.
Molecular adsorbent recirculating system (MARS) combines the conventional CRRT monitor or a standard hemodialysis machine with an albumin dialysate circuit. The system is based on the removal of albumin-bound toxins (bile acids and nitric oxide) and water-soluble cytokines (IL-6 and TNF-α) to stabilize liver function and improve organ damage.
The MARS system consists of an albumin-impermeable membrane separating the patient’s blood from the albumin dialysate solution. The free albumin in the dialysate attracts and binds the liver toxins in the patient’s blood. The albumin dialysate, in its turn, is regenerated by a low flux dialysis filter and two adsorber cartridges, one filled with activated charcoal, the other with an anion exchanger resin. The regenerated albumin solution is then ready for new uptake of toxins from the blood, entering the circuit through a high permeability filter to undergo standard dialysis to remove water-soluble toxins.
Some studies have reported better survival rates in patients treated with MARS compared to conventional CRRT, but overall survival remains very poor (37% at 7 d and 25% at 30 d). The main factor affecting survival is the patient’s clinical status before treatment[75,76].
In 2000, a trial by Mitzner et al[75] assessed survival rates in 13 patients with type I HRS. The eight patients treated with MARS had significantly better survival rates at 30 d than patients receiving standard medical therapy. By contrast, the randomized RELIEF study failed to show any significant differences in terms of survival in 189 patients treated with MARS vs standard medical therapy even though some benefit was noted in the management of encephalopathy in patients with type I HRS who underwent MARS[77].
After a one-year follow-up, Donati et al[78] reported that among 64 patients treated with MARS, the best survival rates were found in the 11 patients who subsequently underwent liver transplant. The same authors observed an improvement in both systolic and diastolic blood pressure in 5 patients with type 2 HRS treated with MARS and standard medical therapy.
The Prometheus system consists of a primary circuit (plasma filter and dialyzer) and a secondary circuit (adsorbent filters to remove bilirubin) for the combined removal of toxin albumin-bound and water-soluble molecules using a fractionated plasma separation and adsorption (FPSA) system. Unlike MARS, the plasma is separated from the blood through a high cut-off point polysulfone membrane (250 kDa, albumin permeable) and purified from the albumin-bound toxins by direct adsorption on resin-containing cartridges. The purified plasma is then returned to the blood circuit through a high efficiency dialyzer to remove water-soluble toxins.
The HELIOS study on 179 patients with liver failure treated with standard medical therapy vs extracorporeal treatment showed no significant advantage in terms of overall survival except in the subgroup of patients with type I HRS treated with FPSA who had a significant survival benefit[79].
Liver transplantation remains the treatment of choice in HRS patients despite its mortality rate which is particularly high in patients with type I HRS whose survival is so poor that many die while awaiting transplant.
Recovery of renal function is not universal: Marik et al[80], in a study on 28 patients, noted a complete recovery of kidney function in only 58% of transplanted patients, a partial recovery in 15% and no recovery in 25% (observation time 110 d). Renal sodium excretion, serum creatinine and neurohormonal levels may normalize within a month whereas renovascular resistance may take more than a year to return to normal after transplantation[81,82].
Organ allocation is mainly based on the MELD score, a system devised to stratify disease severity on the basis of laboratory parameters (serum creatinine, bilirubin and INR) to assign organs according to the so-called sickest first policy[83].
Considering all liver transplant recipients, those with HRS are more exposed to post-transplant complications, at greater risk of developing CKD and have a shorter overall survival[84,85]. Those patients who fail to recover renal function and need to continue hemodialysis have an even worse survival rate (70% mortality at one year)[86].
RRT prior to liver transplant is an important predictive factor. Patients undergoing hemodialysis for more than eight weeks have a markedly reduced probability of renal recovery and a combined liver-kidney transplant is recommended in these cases[87,88] .
Vasopressor treatment of HRS before liver transplant does not seem to affect subsequent patient outcome[89]. Nonetheless, Angeli et al[83] reported that liver transplantation may be delayed in patients treated with vasopressors following a response to treatment and hence an improvement in clinical and hemodynamic status. This paradoxical situation must be avoided and the clinical criteria adopted to establish the priority of patients on the waiting list for transplant (first and foremost the MELD score) must always refer to the patient’s initial condition and not to the status reached after treatment.
There are no specific recommendations as to post-transplant immunosuppressive therapy, but it may be advisable to delay the start of cyclosporine or tacrolimus to 48-72 h after transplantation to enhance renal recovery as suggested by Guevara and Arroyo[90].
HRS is a life-threatening complication arising in patients with liver cirrhosis and triggered by a series of complex hemodynamic and neurohormonal changes linked to the liver disease. The condition carries a very poor prognosis and high morbidity and mortality rates.
Recent years have seen a reduction in HRS prevalence and an improvement in patient outcome probably reflecting a better understanding of HRS pathophysiology and advances in therapeutic strategies.
Treatment consists of medical management (mainly based on vasopressor administration), surgery (TIPS) or instrumental therapies (e.g., renal replacement and liver support systems). Although the therapeutic armamentarium at our disposal will control the syndrome and obtain temporary remission, there is no guarantee of disease resolution.
The only effective treatment offering patients the hope of complete recovery is liver transplantation or combined kidney-liver transplant in selected cases. The decision to embark on transplantation must be carefully assessed in HRS patients considering all the potential factors likely to influence transplant surgery and its outcome.
P- Reviewer: Kelesidis T, Nechifor G, Trimarchi H, Trumper L, Yorioka N S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] [Cited in This Article: ] |
| 2. | Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013;38:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Liangpunsakul S, Agarwal R. Renal failure in cirrhosis: is it time to change the diagnosis and classification? Am J Nephrol. 2013;38:342-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 367] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 6. | Pillebout E. Hepatorenal syndrome. Nephrol Ther. 2014;10:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Wadei HM. Hepatorenal syndrome: a critical update. Semin Respir Crit Care Med. 2012;33:55-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 484] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Francés R, González-Navajas JM, Zapater P, Muñoz C, Caño R, Pascual S, Santana F, Márquez D, Pérez-Mateo M, Such J. Translocation of bacterial DNA from Gram-positive microorganisms is associated with a species-specific inflammatory response in serum and ascitic fluid of patients with cirrhosis. Clin Exp Immunol. 2007;150:230-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675-G685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 11. | Oliver JA, Verna EC. Afferent mechanisms of sodium retention in cirrhosis and hepatorenal syndrome. Kidney Int. 2010;77:669-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Fagundes C, Ginès P. Hepatorenal syndrome: a severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis. 2012;59:874-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 13. | Chayanupatkul M, Liangpunsakul S. Cirrhotic cardiomyopathy: review of pathophysiology and treatment. Hepatol Int. 2014;8:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (1)] |
| 14. | Mocarzel L, Lanzieri P, Nascimento J, Peixoto C, Ribeiro M, Mesquita E. Hepatorenal syndrome with cirrhotic cardiomyopathy: case report and literature review. Case Reports Hepatol. 2015;2015:573513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Fede G, D’Amico G, Arvaniti V, Tsochatzis E, Germani G, Georgiadis D, Morabito A, Burroughs AK. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56:810-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 584] [Cited by in F6Publishing: 485] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, Galeras JA, Giménez MD, Santos J, Cirera I. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005;68:362-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1102] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 20. | Hkecer R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956;271:1121-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 170] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Koppel MH, Coburn JW, Mims MM, Goldstein H, Boyle JD, Rubini ME. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 205] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1084] [Cited by in F6Publishing: 1002] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 23. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-S89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, Salinas F, Donà S, Fagiuoli S, Sticca A. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Moore K. Acute kidney injury in cirrhosis: a changing spectrum. Hepatology. 2013;57:435-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Nadim MK, Kellum JA, Davenport A, Wong F, Davis C, Pannu N, Tolwani A, Bellomo R, Genyk YS. Hepatorenal syndrome: the 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16:R23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, Graupera I, Ariza X, Pereira G, Alfaro I. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Arroyo V. Acute kidney injury (AKI) in cirrhosis: should we change current definition and diagnostic criteria of renal failure in cirrhosis? J Hepatol. 2013;59:415-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Seo YS, Jung ES, An H, Kim JH, Jung YK, Kim JH, Yim HJ, Yeon JE, Byun KS, Kim CD. Serum cystatin C level is a good prognostic marker in patients with cirrhotic ascites and normal serum creatinine levels. Liver Int. 2009;29:1521-1527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 32. | Sharawey MA, Shawky EM, Ali LH, Mohammed AA, Hassan HA, Fouad YM. Cystatin C: a predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol Int. 2011;5:927-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 34. | Møller S, Krag A, Bendtsen F. Kidney injury in cirrhosis: pathophysiological and therapeutic aspects of hepatorenal syndromes. Liver Int. 2014;34:1153-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 438] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 36. | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 37. | Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, Adkins SH, Sise ME, Oliver JA, Radhakrishnan J. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57:2362-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, Pereira G, Rodríguez E, Garcia E, Prado V. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Barreto R, Elia C, Solà E, Moreira R, Ariza X, Rodríguez E, Graupera I, Alfaro I, Morales-Ruiz M, Poch E. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol. 2014;61:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Fabrizi F, Aghemo A, Messa P. Hepatorenal syndrome and novel advances in its management. Kidney Blood Press Res. 2013;37:588-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Davenport A, Ahmad J, Al-Khafaji A, Kellum JA, Genyk YS, Nadim MK. Medical management of hepatorenal syndrome. Nephrol Dial Transplant. 2012;27:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, Angeli P, Ruiz-Del-Arbol L, Planas R, Solà R. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 415] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 43. | García-Compean D, Blanc P, Larrey D, Daures JP, Hirtz J, Mendoza E, Maldonado H, Michel H. Treatment of cirrhotic tense ascites with Dextran-40 versus albumin associated with large volume paracentesis: a randomized controlled trial. Ann Hepatol. 2002;1:29-35. [PubMed] [Cited in This Article: ] |
| 44. | Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 45. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1132] [Cited by in F6Publishing: 972] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 46. | Fernández J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, Lacy AM, Ginès P, Arroyo V. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Barbano B, Sardo L, Gigante A, Gasperini ML, Liberatori M, Giraldi GD, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 49. | Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, Amodio P, Sticca A, Caregaro L, Maffei-Faccioli A. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 316] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 50. | Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 51. | Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 52. | Angeli P. Review article: prognosis of hepatorenal syndrome--has it changed with current practice? Aliment Pharmacol Ther. 2004;20 Suppl 3:44-6; discussion 47-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 54. | Fabrizi F, Dixit V, Martin P. Meta-analysis: terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol Ther. 2006;24:935-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Sugumaran A, Lougher E, Czajkowski M, Yeoman A. Reduction of serum sodium (Na) in patients treated with terlipressin for variceal bleeding (VB) and hepatorenal syndrome (HRS). Gut. 2014;63:A87-A88. [DOI] [Cited in This Article: ] |
| 56. | Fabrizi F, Dixit V, Messa P, Martin P. Terlipressin for hepatorenal syndrome: A meta-analysis of randomized trials. Int J Artif Organs. 2009;32:133-140. [PubMed] [Cited in This Article: ] |
| 57. | Duvoux C, Zanditenas D, Hézode C, Chauvat A, Monin JL, Roudot-Thoraval F, Mallat A, Dhumeaux D. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36:374-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 58. | Wadei HM, Gonwa TA. Hepatorenal syndrome in the intensive care unit. J Intensive Care Med. 2013;28:79-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Ghosh S, Choudhary NS, Sharma AK, Singh B, Kumar P, Agarwal R, Sharma N, Bhalla A, Chawla YK, Singh V. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int. 2013;33:1187-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Prabhu MV, Sukanya B, Santosh Pai BH, Reddy S. The hepatorenal syndrome - a review. G Ital Nefrol. 2014;31. [PubMed] [Cited in This Article: ] |
| 61. | Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 276] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 65. | Pipili C, Cholongitas E. Renal dysfunction in patients with cirrhosis: Where do we stand? World J Gastrointest Pharmacol Ther. 2014;5:156-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Keller F, Heinze H, Jochimsen F, Passfall J, Schuppan D, Büttner P. Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): the role of hemodialysis. Ren Fail. 1995;17:135-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Wilkinson SP, Weston MJ, Parsons V, Williams R. Dialysis in the treatment of renal failure in patients with liver disease. Clin Nephrol. 1977;8:287-292. [PubMed] [Cited in This Article: ] |
| 68. | Davenport A. Renal replacement therapy in the patient with acute brain injury. Am J Kidney Dis. 2001;37:457-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Witzke O, Baumann M, Patschan D, Patschan S, Mitchell A, Treichel U, Gerken G, Philipp T, Kribben A. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19:1369-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. Renal replacement therapy and orthotopic liver transplantation: the role of continuous veno-venous hemodialysis. Transplantation. 2001;71:1424-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am. 2009;93:855-869, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Patel S, Wendon J. Regional citrate anticoagulation in patients with liver failure--time for a rethink? Crit Care. 2012;16:153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Pipili C, Polydorou A, Pantelias K, Korfiatis P, Nikolakopoulos F, Grapsa E. Improvement of hepatic encephalopathy by application of peritoneal dialysis in a patient with non-end-stage renal disease. Perit Dial Int. 2013;33:213-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1:915-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 75. | Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 76. | Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Nöldge-Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol. 2001;12 Suppl 17:S75-S82. [PubMed] [Cited in This Article: ] |
| 77. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 78. | Donati G, La Manna G, Cianciolo G, Grandinetti V, Carretta E, Cappuccilli M, Panicali L, Iorio M, Piscaglia F, Bolondi L. Extracorporeal detoxification for hepatic failure using molecular adsorbent recirculating system: depurative efficiency and clinical results in a long-term follow-up. Artif Organs. 2014;38:125-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van Vlierberghe H, Escorsell A. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782-789.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 80. | Marik PE, Gayowski T, Starzl TE. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit Care Med. 2005;33:1254-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 81. | Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, Rimola A, Bosch J, Rodés J. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Piscaglia F, Zironi G, Gaiani S, Mazziotti A, Cavallari A, Gramantieri L, Valgimigli M, Bolondi L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: a long-term prospective study. Hepatology. 1999;30:58-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Angeli P, Gines P. Hepatorenal syndrome, MELD score and liver transplantation: an evolving issue with relevant implications for clinical practice. J Hepatol. 2012;57:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934-1939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 376] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 85. | Lee JP, Heo NJ, Joo KW, Yi NJ, Suh KS, Moon KC, Kim SG, Kim YS. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant. 2010;25:2772-2785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 86. | Ruiz R, Barri YM, Jennings LW, Chinnakotla S, Goldstein RM, Levy MF, McKenna GJ, Randall HB, Sanchez EQ, Klintmalm GB. Hepatorenal syndrome: a proposal for kidney after liver transplantation (KALT). Liver Transpl. 2007;13:838-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transplant. 2006;21:478-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Gerbes AL, Gülberg V, Waggershauser T, Holl J, Reiser M. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology. 1998;28:683-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Restuccia T, Ortega R, Guevara M, Ginès P, Alessandria C, Ozdogan O, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 139] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 90. | Guevara M, Arroyo V. Hepatorenal syndrome. Expert Opin Pharmacother. 2011;12:1405-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |