Copyright
©The Author(s) 2016.
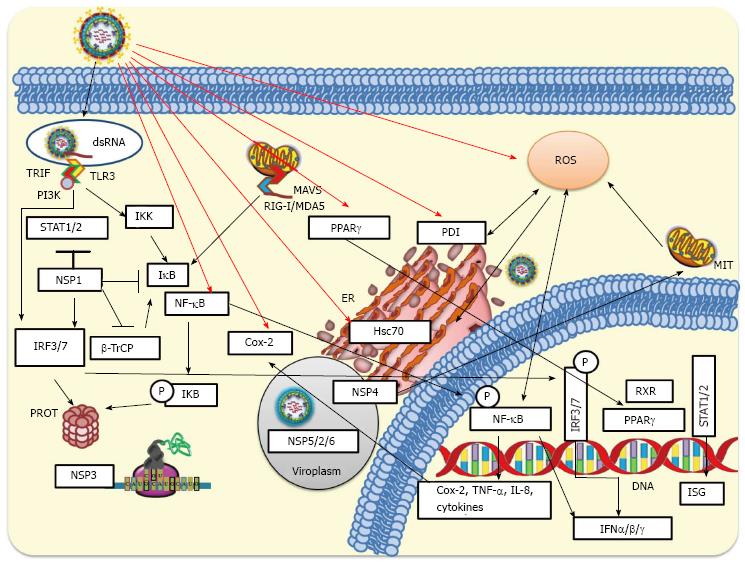
Figure 3 Cellular innate response to rotavirus infection.
During rotavirus internalization viral nucleic acid may be exposed and recognized by either Toll-like receptors (TLR3) or intracellular RIG-I-like receptors (RLRs). Activated RLRs can bind and activate mitochondrial antiviral-signaling protein (MAVS), which recruits a signaling complex needed to activate cytoplasmic transcription factors including interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB). On the other hand, activation of endosomal TLR3 facilitates the adaptor TRIF recruitment, which allows the recruitment of signaling molecules such as IKKs that phosphorylate IRF3 or NF-κB. Phosphorylated IRF3 is dimerized and then translocated to the nucleus. Signaling pathways induced by rotavirus infection produce phosphorylation of IκB (inhibitor of NF-κB) and its subsequent ubiquitination and proteasomal degradation mediated by SCFβ-TrCP E3 ligase. This signaling pathway leads to NF-κB translocation to the nucleus where, jointly with IRF3 and IRF7, binds to the interferon (IFN)-β promoter for transcription of IFN-β mRNA. Rotavirus can early counteract signaling pathways of innate response by NSP1-mediated degradation of IRF3 and IRF7. NSP1 encoded by some rotavirus strains can target SCFβ-TrCP for proteasomal degradation, whereas NSP1 from other strains has been implicated in the direct inhibition of the IFN-mediated STAT1 activation. NSP3 can interfere with the translation of cellular-encoded proteins including those induced by the IFN signaling. The viroplasm, which includes some viral non-structural proteins (NSP2/5/6), can protect viral RNAs from being recognized by some pattern-recognition receptors (RIG-I, MDA-5, among others) involved in antiviral response. MIT, ER and PROT are indicated. IKK: IκB kinase; MIT: Mitochondria; ER: Endoplasmic reticulum; PROT: Proteasome; TNF: Tumor necrosis factor.
- Citation: Guerrero CA, Acosta O. Inflammatory and oxidative stress in rotavirus infection. World J Virol 2016; 5(2): 38-62
- URL: https://www.wjgnet.com/2220-3249/full/v5/i2/38.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i2.38