Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.89469
Peer-review started: November 2, 2023
First decision: December 19, 2023
Revised: December 19, 2023
Accepted: January 18, 2024
Article in press: January 18, 2024
Published online: March 25, 2024
Hepatitis C is the leading cause of chronic liver disease worldwide and it significantly contributes to the burden of hepatocellular carcinoma (HCC). However, there are marked variations in the incidence and mortality rates of HCC across different geographical regions. With the advent of new widely available treatment modalities, such as direct-acting antivirals, it is becoming increasingly imperative to understand the temporal and geographical trends in HCC mortality associated with Hepatitis C. Furthermore, gender disparities in HCC mortality related to Hepatitis C are a crucial, yet underexplored aspect that adds to the disease's global impact. While some studies shed light on gender-specific trends, there is a lack of comprehensive data on global and regional mortality rates, particularly those highlighting gender disparities. This gap in knowledge hinders the development of targeted interventions and resource allocation strategies.
To understand the global and regional trends in Hepatitis C-related HCC mortality rates from 1990 to 2019, along with gender disparities.
We utilized the Global Burden of Disease database, a comprehensive repository for global health metrics to age-standardized mortality rates due to Hepatitis C-related HCC from 1999 to 2019. Rates were evaluated per 100000 population and assessed by World Bank-defined regions. Temporal trends were determined using Joinpoint software and the Average Annual Percent Change (AAPC) method, and results were reported with 95% confidence intervals (CI).
From 1990 to 2019, overall, there was a significant decline in HCC-related mortality rates with an AAPC of -0.80% (95%CI: -0.83 to -0.77). Females demonstrated a marked decrease in mortality with an AAPC of -1.06% (95%CI: -1.09 to -1.03), whereas the male cohort had a lower AAPC of -0.52% (95%CI: -0.55 to -0.48). Regionally, East Asia and the Pacific demonstrated a significant decline with an AAPC of -2.05% (95%CI: -2.10 to -2.00), whereas Europe and Central Asia observed an uptrend with an AAPC of 0.72% (95%CI: 0.69 to 0.74). Latin America and the Caribbean also showed an uptrend with an AAPC of 0.06% (95%CI: 0.02 to 0.11). In the Middle East and North Africa, the AAPC was non-significant at 0.02% (95%CI: -0.09 to 0.12). North America, in contrast, displayed a significant upward trend with an AAPC of 2.63% (95%CI: 2.57 to 2.67). South Asia (AAPC -0.22%, 95%CI: -0.26 to -0.16) and Sub-Saharan Africa (AAPC -0.14%, 95%CI: -0.15 to -0.12) trends significantly declined over the study period.
Our study reports disparities in Hepatitis C-related HCC mortality between 1999 to 2019, both regionally and between genders. While East Asia and the Pacific regions showed a promising decline in mortality, North America has experienced a concerning rise in mortality. These regional variations highlight the need for healthcare policymakers and practitioners to tailor public health strategies and interventions. The data serves as a call to action, particularly for regions where mortality rates are not improving, emphasizing the necessity for a nuanced, region-specific approach to combat the global challenge of HCC secondary to Hepatitis C.
Core Tip: Hepatitis C virus (HCV) remains a crucial precursor for hepatocellular carcinoma (HCC), accounting for a significant proportion of HCC-related mortalities. Our study focused on trends from 1999 to 2019 and offers an extensive temporal analysis on the mortality rates in patients with HCV-related HCC. The data highlighted that despite advances in antiviral treatments for HCV, the mortality rates in HCC have not seen a corresponding decline. We also identified noticeable trends relating to gender, providing insights into demographic groups that are disproportionately affected. This study emphasizes the need for targeted interventions to reduce mortality rates in HCV-associated HCC, despite advancements in HCV treatment.
- Citation: Ali H, Vikash F, Moond V, Khalid F, Jamil AR, Dahiya DS, Sohail AH, Gangwani MK, Patel P, Satapathy SK. Global trends in hepatitis C-related hepatocellular carcinoma mortality: A public database analysis (1999-2019). World J Virol 2024; 13(1): 89469
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/89469.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.89469
In the late 20th century, there was a significant shift in the etiology of chronic liver disease due to the advent of Hepatitis C infection, which also contributed to a rising trend in the incidence of liver-related malignancies[1]. Data from the World Health Organization (WHO) indicates that globally, around 58 million individuals are infected with the hepatitis C virus (HCV). Each year, there are about 1.5 million new cases of HCV, and the virus is responsible for roughly 290000 fatalities annually[2]. Based on 2020 data, hepatocellular carcinoma (HCC) ranks as the sixth most frequently diagnosed cancer globally and stands as the third leading cause of cancer-related deaths[3]. In Africa and East Asia, HBV infection is the predominant cause of HCC, responsible for up to 60% of cases. Conversely, in Western countries, chronic HCV infection emerges as the primary etiological factor for HCC, with about 34% of HCC cases in the United States (USA) attributed to it[4].
The annual incidence of HCC in patients with hepatitis C ranges from about 1-8%, being the highest in Japan (4%-8%), followed by Italy (2%-4%), and USA (1.4%)[5]. Various studies suggest that HCC might be the first complication to develop and a more common cause of death in patients with HCV-associated cirrhosis[6]. Most cases of HCC manifest in individuals with cirrhosis, which serves as an independent risk factor for developing HCC, irrespective of the underlying cause of the liver disease. Data suggests that about one-third of patients with cirrhosis will develop liver cancer during their lifetimes[7]. The annual incidence of HCC among cirrhotic patients ranges between 1%-8%, with specific rates varying based on the type of liver disease, and estimates indicate that patients infected with HCV face a 15 to 20 times higher risk of developing HCC. In those with cirrhosis, the annual incidence of HCC is projected to be between 1% and 4% over a period of 30 years[8]. In 2012, data indicated that around 170000 new cancer cases, which is approximately 7.8% of all the newly diagnosed cancers, were attributed to the HCV[9]. Over the last ten years, deaths attributable to HCV-related HCC have increased by 21.1%; however, at the same time, deaths from HCC secondary to causes other than HCV and alcohol have remained stable[10]. Hence, this escalating global burden of HCV infection-related HCC has gained attention among clinicians, public health professionals, and researchers.
There has been variability in the incidence of HCV-related HCC, based on ethnicity and geographic location. HCV is recognized as the primary cause of HCC in regions including the USA, Europe, Japan, and South America[10]. Delving deeper into country-specific data, the prevalence of HCV in Japan has been estimated to be 3%, and an estimated 85% of patients with HCC are infected with HCV[11]. In the USA, where the prevalence of HCV is about 1.8% of the population, around 50%-60% of HCC patients are HCV-positive[12]. Analyzing the demographic distribution within the USA, there are distinct variations of HCC by ethnicity and age. Particularly, Hispanic individuals and those born between 1945 and 1965 are at the highest risk of HCC[13]. A comprehensive study involving 150000 HCV-infected USA veterans revealed that the Hispanic demographic reported the most substantial yearly HCC incidence at 7.8%. This heightened incidence is believed to correlate with the increased prevalence of nonalcoholic fatty liver disease within this group[14]. Another study from a comparable cohort highlighted a 2.5-fold surge in HCC incidence, with a threefold increase in mortality since 2001, even after the introduction of direct-acting antivirals (DAAs)[15].
Despite the recent advancement in the development of antiviral therapies, including DAAs[16], which have, to an extent, revolutionized the treatment of HCV, there persists an ongoing debate on the long-term impact of the virus on liver health. Adequate treatment helps cure the active infection, but the possibility of progression to cirrhosis leading to HCC persists in some patients. Hence, it makes understanding the global mortality trends in HCV-related HCC of high academic interest and critical importance for public health policy and intervention strategies. In 2016, the World Health Assembly proposed eliminating viral hepatitis as a public health threat[17]. Still, most countries need to meet their expected goals by 2020 due to the impact of the coronavirus disease 2019 pandemic and various other factors[18]. Consequently, in 2021, the WHO established guidance featuring absolute incidence targets, specifying the number of new HCV infections per 100000 persons each year[19].
In our analysis, we aim to examine the change in trends in regional and gender-specific variances in mortality rates due to HCC secondary to hepatitis C. We aim to identify disparities that inform targeted interventions for at-risk populations. Prior studies have mainly analyzed global liver cancer trends without focusing on specific etiologies such as HCV. To our knowledge, no prior study has estimated the risk of HCV-related HCC mortality and HCC trends over time, covering all World Bank regions. This article aims to provide a comprehensive review of current mortality trends in Hepatitis C-related HCC. It will provide a better understanding of the global mortality burden and facilitate designs for policymakers by providing targeted measures to mitigate the impact of the increasingly prevalent malignancy.
The primary data source for this study was the Global Burden of Disease (GBD) database, a comprehensive repository for global health metrics. Initiated in the early 1990s, the GBD database is continually updated to offer the most current insights into the global prevalence, incidence, and impact of diseases[20]. Compiled by the Institute for Health Metrics and Evaluation, the database strictly adheres to the Guidelines for Accurate and Transparent Health Estimates Reporting. The GBD database encompasses data from numerous countries and territories, capturing various health-related metrics, including mortality. Our analysis was divided by World Bank-defined regions, including Sub-Saharan Africa, East Asia and Pacific, Europe and Central Asia, Latin America and the Caribbean, Middle East and North Africa, and South Asia to provide a more comprehensive geographical perspective.
This study focused on mortality related to HCC due to Hepatitis C to comprehensively understand the disease's long-term trends and status. Age-standardized estimates were employed to adjust for differences in the age distribution of different populations, aligning them to a standardized reference world population. This adjustment enabled the comparison of disease burdens across diverse populations. As in prior studies, rates were reported per 100000 population and were age-standardized to facilitate comparisons across different demographic structures[21].
We examined age-standardized mortality rates per 100000 population to analyze hepatitis C-related HCC mortality rates. Joinpoint software assessed temporal trends in the Average Percent Change (APC), representing the change in mortality during a specific period. Joinpoint identified time points where the trend changed significantly using a Monte Carlo permutation test and a t-test. Log-linear regression models were fitted to evaluate trends in age-standardized mortality rates. To enhance the reliability of our analysis, especially in the context of uncertain data distributions, we employed Joinpoint's Empirical Quantile Confidence Interval method. This approach provides robust and conservative 95% confidence intervals for APC, Average Annual Percent Change (AAPC), and the location of Joinpoints (tau). Unlike traditional methods that yield P values, these confidence intervals directly reflect the measurement level of the data, offering a more intuitive understanding of result reliability and significance[22]. We limited our models to a maximum of three Joinpoints to minimize bias. Statistical significance was determined based on these 95% confidence intervals, as the Empirical Quantile method does not compute traditional P values but offers a more reliable measure of significance[23].
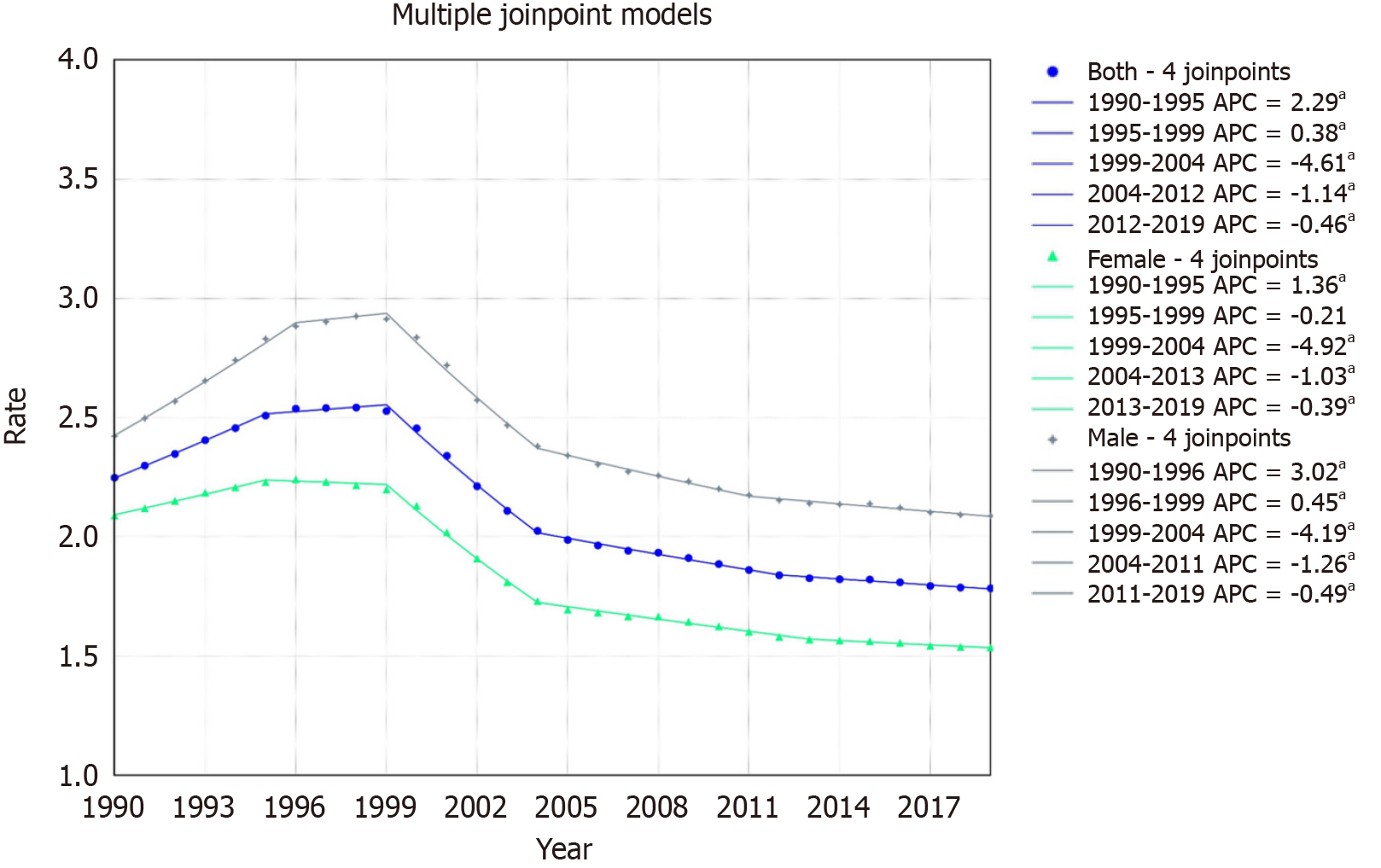
The overall mortality rate declined from 2.25/100000 in 1999 to 1.78/100000 in 2019, with an AAPC of -0.80% (95%CI:
For females, the rate declined from 2.08/100000 in 1999 to 1.53/100000 in 2019, with an AAPC of -1.06% (95%CI: -1.09 to -1.03). The trends showed an increase from 1990-1995 (APC: 1.36, 95%CI: 1.11 to 1.65) with subsequent declines, notably from 1999-2004 (APC: -4.92, 95%CI: -5.07 to -4.75) (Figure 1).
For males, the rate declined from 2.42/100000 in 1999 to 2.08/100000 in 2019, with an AAPC of -0.52% (95%CI: -0.55 to
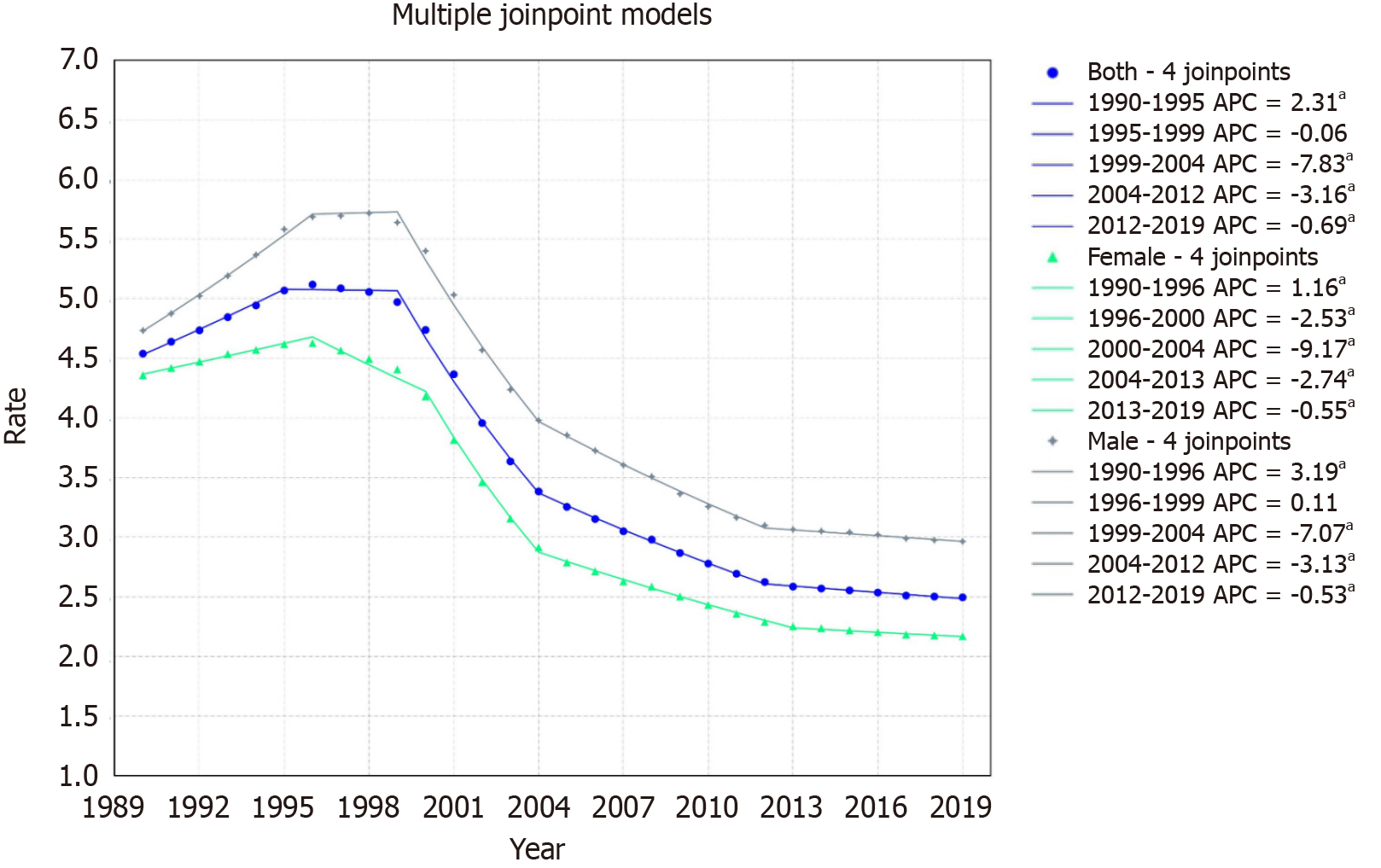
The rate declined from 4.54/100000 to 2.50/100000 with an AAPC of -2.05% with a 95%CI of -2.10 to -2.00. The mortality rate displayed five segments: An increase from 1990-1995 (APC: 2.31, 95%CI: 1.92 to 2.81), a slight drop from 1995-1999 (APC: -0.06, 95%CI: -0.62 to 0.42), followed by sharp declines from 1999-2004 (APC: -7.83, 95%CI: -8.08 to -7.57), 2004-2012 (APC: -3.16, 95%CI: -3.38 to -2.98), and 2012-2019 (APC: -0.69, 95%CI: -0.94 to -0.34) (Figure 2).
For females, the rate declined from 4.35/100000 in 1999 to 2.19/100000 in 2019, with an AAPC of -2.39 (95%CI: -2.43 to
For males, the rate declined from 4.73/100000 in 1999 to 2.96/100000 in 2019, with an AAPC of -1.60 (95%CI: -1.64 to
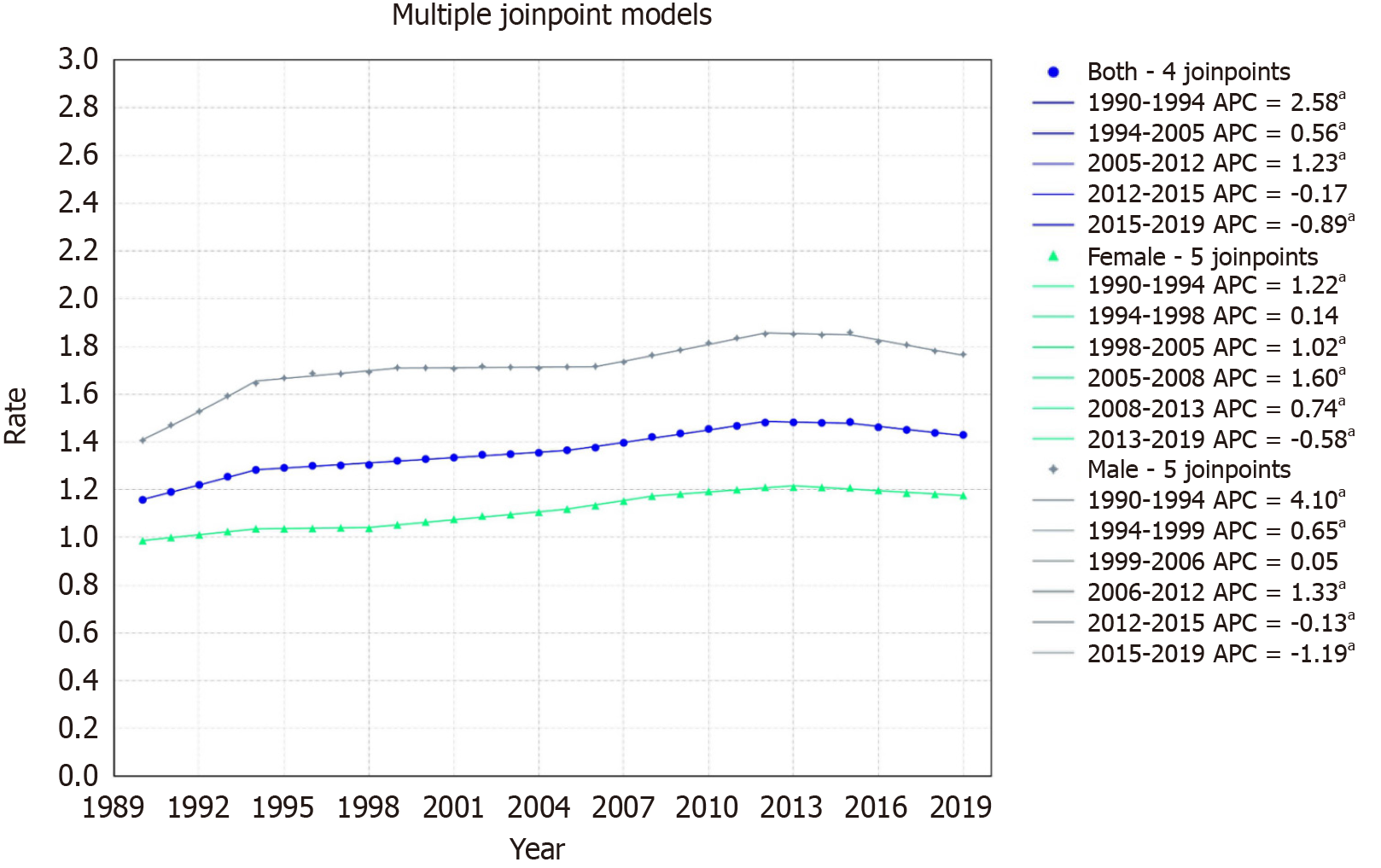
The overall population's mortality rate increased from 1.16/100000 in 1999 to 1.43/100000 in 2019, with an AAPC of 0.72, with a 95%CI of 0.69 to 0.74. The mortality rate exhibited five distinct segments: An increase from 1990-1994 (APC: 2.58, 95%CI: 2.44 to 2.73), a steady rise from 1994-2005 (APC: 0.56, 95%CI: 0.50 to 0.61), a further increase from 2005-2012 (APC: 1.23, 95%CI: 1.12 to 1.38), a negligible decline from 2012-2015, and a drop from 2015-2019 (APC: -0.89, 95%CI: -1.19 to
For females, the rate increased from 0.98/100000 in 1999 to 1.17/100000 in 2019, with an AAPC of 0.60% (95%CI: 0.59 to 0.62). Mortality trends indicated an increase from 1990-1994 (APC: 1.22, 95%CI: 1.07 to 1.39) with subsequent varied changes, a notable rise from 1998-2005 (APC: 1.02, 95%CI: 0.90 to 1.10), a significant increase from 2005-2008 (APC: 1.60, 95%CI: 1.33 to 1.73), a steady rise from 2008-2013 (APC: 0.74, 95%CI: 0.59 to 0.82), and a decline from 2013-2019 (APC:
For males, the rate increased from 1.41/100000 in 1999 to 1.76/100000 in 2019, with an AAPC of 0.77% (95%CI: 0.75 to 0.80). The segments displayed an initial rise from 1990-1994 (APC: 4.10, 95%CI: 3.91 to 4.30), growth from 1994-1999 (APC: 0.65, 95%CI: 0.50 to 0.86), a plateau from 1999-2006, an increase from 2006-2012 (APC: 1.33, 95%CI: 1.20 to 1.54), a slight drop from 2012-2015, and a decline from 2015-2019 (APC: -1.19, 95%CI: -1.50 to -1.01) (Figure 3).
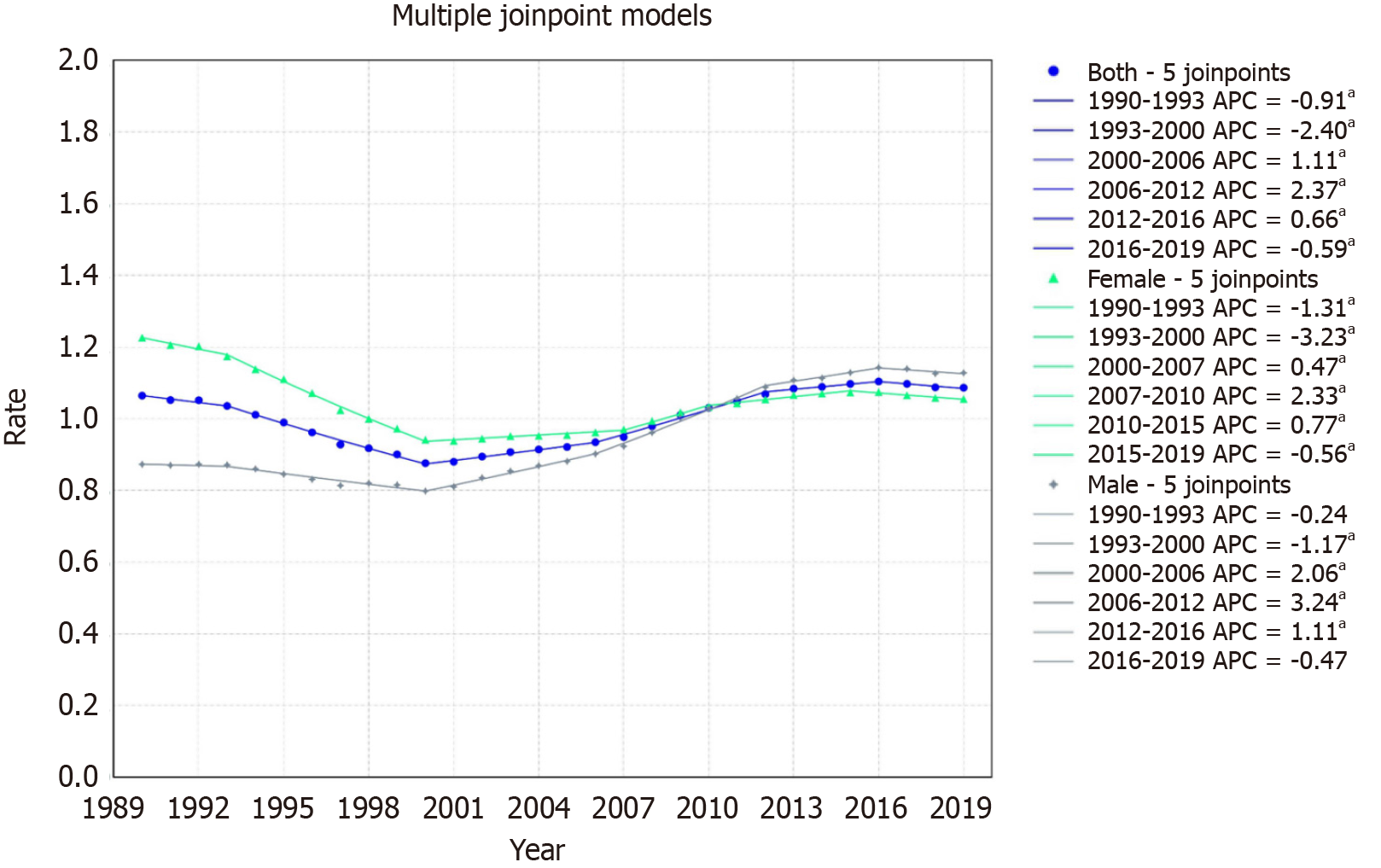
For Latin America & Caribbean, a minor increase was noted from 1.06/100000 in 1999 to 1.09/100000 in 2019 with an AAPC of 0.06 with a 95%CI of 0.02 to 0.11. Mortality trends displayed six segments: An initial decline from 1990-1993 (APC: -0.91, 95%CI: -1.40 to -0.26), a further decrease from 1993-2000 (APC: -2.40, 95%CI: -2.56 to -2.26), an increase from 2000-2006 (APC: 1.11, 95%CI: 0.83 to 1.29), a pronounced rise from 2006-2012 (APC: 2.37, 95%CI: 2.11 to 2.81), a steady increase from 2012-2016 (APC: 0.66, 95%CI: 0.35 to 1.45), and a decline from 2016-2019 (APC: -0.59, 95%CI: -1.34 to -0.12) (Figure 4).
For females, the rate declined from 1.22/100000 in 1999 to 1.05/100000 in 2019, with an AAPC of -0.52% (95%CI: -0.56 to -0.48). The trends displayed six segments: A decline from 1990-1993 (APC: -1.31, 95%CI: -1.86 to -0.79), a sharp decrease from 1993-2000 (APC: -3.23, 95%CI: -3.36 to -3.10), a slight increase from 2000-2007 (APC: 0.47, 95%CI: 0.26 to 0.60), a significant rise from 2007-2010 (APC: 2.33, 95%CI: 1.76 to 2.63), a steady increase from 2010-2015 (APC: 0.77, 95%CI: 0.46 to 1.00), and a decline from 2015-2019 (APC: -0.56, 95%CI: -1.10 to -0.24) (Figure 4).
For males, the rate increased from 0.87/100000 in 1999 to 1.12/100000 in 2019, with an AAPC of 0.88% (95%CI: 0.82 to 0.94). Mortality trends highlighted an initial negligible change from 1990-1993, a decline from 1993-2000 (APC: -1.17, 95%CI: -1.66 to -0.97), a pronounced rise from 2000-2006 (APC: 2.06, 95%CI: 1.52 to 2.41), a further significant increase from 2006-2012 (APC: 3.24, 95%CI: 2.89 to 3.89), an increase from 2012-2016 (APC: 1.11, 95%CI: 0.70 to 2.08), and a slight decline from 2016-2019 (APC: -0.47) though it was not statistically significant (Figure 4).
In the Middle East & North Africa, the rate slightly decreased from 3.81/100000 to 3.74/100000 with an AAPC of 0.02 with a 95%CI of -0.09 to 0.12. The mortality rate presented three segments: An initial decline from 1990-1999 (APC: -1.10, 95%CI: -1.64 to -0.69), a substantial rise from 1999-2011 (APC: 2.10, 95%CI: 1.80 to 2.49), and a decrease from 2011-2019 (APC: -1.77, 95%CI: -2.35 to -1.27) (Figure 5).
For females, the rate declined from 2.36/100000 in 1999 to 2.12/100000 in 2019, with an AAPC of -0.38% (95%CI: -0.44 to -0.33). The trends indicated six segments: A decline from 1990-2000 (APC: -1.79, 95%CI: -2.11 to -1.61), a transient increase from 2000-2003 (APC: 2.58, not statistically significant), a mild rise from 2003-2006 (APC: 0.91, 95%CI: 0.29 to 1.88), a pronounced increase from 2006-2009 (APC: 4.14, 95%CI: 2.91 to 4.73), a decrease from 2009-2015 (APC: -0.87, 95%CI: -1.20 to -0.45), and a significant decline from 2015-2019 (APC: -2.51, 95%CI: -3.39 to -2.00) (Figure 5).
For males, the rate increased from 5.20/100000 in 1999 to 5.25/100000 in 2019, with an AAPC of 0.12% (95%CI: -0.02 to 0.24). The trends showed three segments: An initial decline from 1990-1998 (APC: -1.10, 95%CI: -2.02 to -0.49), a substantial increase from 1998-2011 (APC: 2.04, 95%CI: 1.74 to 2.51), and a decline from 2011-2019 (APC: -1.74, 95%CI: -2.42 to -1.18) (Figure 5).
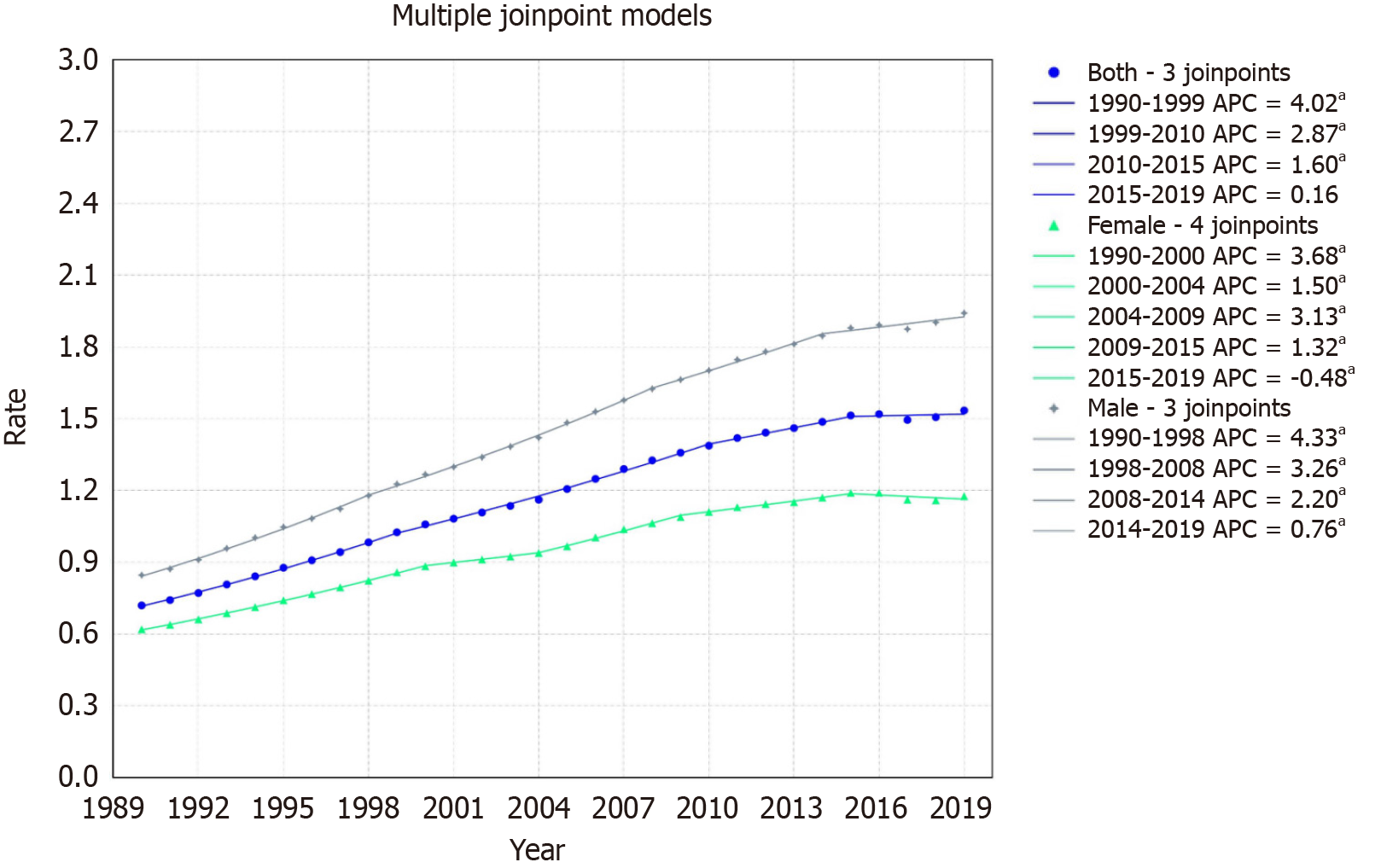
In North America, the mortality rate increased from 0.72/100000 to 1.53/100000 with an AAPC of 2.63, with a 95%CI of 2.57 to 2.67. The mortality trends showcased four distinct segments: A rise from 1990-1999 (APC: 4.02, 95%CI: 3.86 to 4.21), an increase from 1999-2010 (APC: 2.87, 95%CI: 2.74 to 3.01), an uptick from 2010-2015 (APC: 1.60, 95%CI: 1.20 to 2.10), and a minor rise from 2015-2019 (APC: 0.16, not statistically significant) (Figure 6).
For females, the rate increased from 0.61/100000 in 1999 to 1.17/100000 in 2019, with an AAPC of 2.21% (95%CI: 2.18 to 2.25). The mortality trends illustrated five segments: An increase from 1990-2000 (APC: 3.68, 95%CI: 3.58 to 3.79), a rise from 2000-2004 (APC: 1.50, 95%CI: 1.00 to 1.95), a pronounced growth from 2004-2009 (APC: 3.13, 95%CI: 2.83 to 3.69), an increase from 2009-2015 (APC: 1.32, 95%CI: 1.06 to 1.59), and a decline from 2015-2019 (APC: -0.48, 95%CI: -0.97 to -0.09) (Figure 6).
For males, the rate increased from 0.84/100000 in 1999 to 1.94/100000 in 2019, with an AAPC of 2.90% (95%CI: 2.84 to 2.95). The trends presented four segments: An initial rise from 1990-1998 (APC: 4.33, 95%CI: 4.12 to 4.61), growth from 1998-2008 (APC: 3.26, 95%CI: 3.10 to 3.47), an increase from 2008-2014 (APC: 2.20, 95%CI: 1.81 to 2.59), and an uptick from 2014-2019 (APC: 0.76, 95%CI: 0.17 to 1.10) (Figure 6).
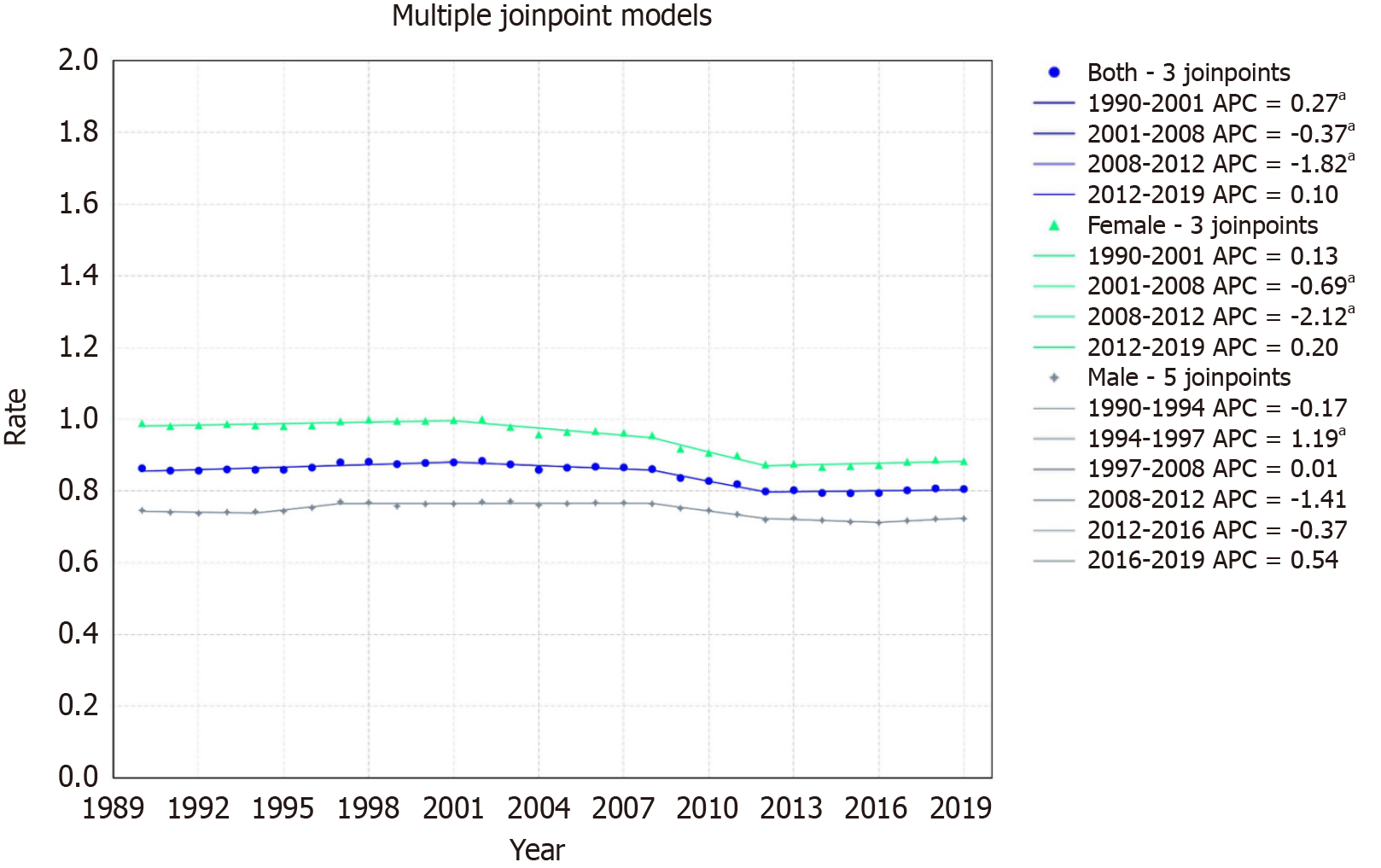
In South Asia, rates decreased from 0.86/100000 to 0.81/100000 with an AAPC of -0.22 with a 95%CI of -0.26 to -0.16. The mortality trends revealed four segments: An initial increase from 1990-2001 (APC: 0.27, 95%CI: 0.14 to 0.54), a decrease from 2001-2008 (APC: -0.37, 95%CI: -0.77 to -0.05), a sharp decline from 2008-2012 (APC: -1.82, 95%CI: -2.54 to -1.16), and a minor uptick from 2012-2019 (APC: 0.10, 95%CI: -0.17 to 0.57) (Figure 7).
For females, the rate declined from 0.98/100000 in 1999 to 0.88/100000 in 2019, with an AAPC of -0.37% (95%CI: -0.42 to -0.31). The mortality trends showcased four segments: A slight increase from 1990-2001 (APC: 0.13, 95%CI: -0.01 to 0.39), a decline from 2001-2008 (APC: -0.69, 95%CI: -1.07 to -0.29), a sharp drop from 2008-2012 (APC: -2.12, 95%CI: -2.94 to -1.40), and a mild rise from 2012-2019 (APC: 0.20, 95%CI: -0.11 to 0.67) (Figure 7).
For males, the rate declined from 0.75/100000 in 1999 to 0.72/100000 in 2019 with an AAPC of -0.09 (95%CI: -0.13 to
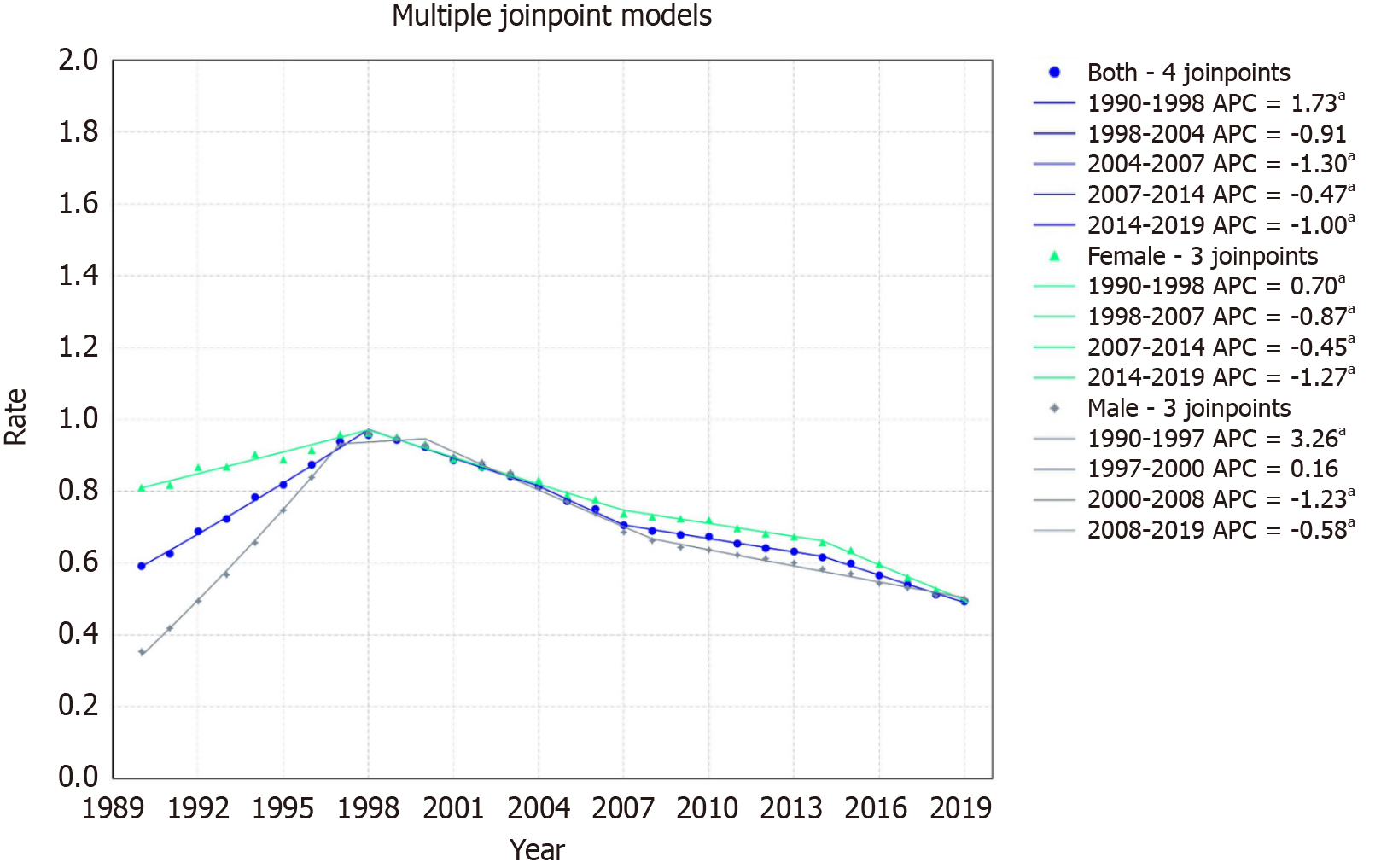
Sub-Saharan Africa mortality rates decreased from 1.30/100000 to 1.25/100000 with an AAPC of -0.14 with a 95%CI of
For females, the rate declined from 1.40/100000 in 1999 to 1.24/100000 in 2019, with an AAPC of -0.41% (95%CI: -0.43 to -0.38). The trends demonstrated four segments: An increase from 1990-1998 (APC: 0.70, 95%CI: 0.60 to 0.80), followed by declines from 1998-2007 (APC: -0.87, 95%CI: -1.05 to -0.79), 2007-2014 (APC: -0.45, 95%CI: -0.58 to -0.07), and 2014-2019 (APC: -1.27, 95%CI: -1.53 to -1.08) (Figure 8).
For males, the rate increased from 1.17/100000 in 1999 to 1.25/100000 in 2019, with an AAPC of 0.23% (95%CI: 0.21 to 0.26). The patterns illustrated four segments: A pronounced rise from 1990-1997 (APC: 3.26, 95%CI: 3.15 to 3.40), followed by minor growth from 1997-2000 (APC: 0.16, 95%CI: -0.23 to 0.48), a decline from 2000-2008 (APC: -1.23, 95%CI: -1.44 to
The results of our study show an overall decline in the mortality rates of patients with hepatitis C-related HCC over the last two decades. Notably, females exhibited a remarkable decrease in mortality compared to males. Regionally, East Asia and the Pacific displayed a significant decline in mortality, while Europe and Central Asia witnessed an upward trend. Latin America and the Caribbean also experienced an increase in mortality rates. However, no significant difference was observed in the Middle East and North Africa. North America exhibited a notable upward trend. South Asia and Sub-Saharan Africa significantly declined throughout the study period. This raises the hope of identifying areas for implementing more targeted resources. Despite some progress, multiple challenges remain in meeting the WHO 2030 goal of eliminating viral hepatitis[24].
Globally, over 170 million people are affected by chronic HCV, with 50%-80% of these cases eventually leading to cirrhosis and HCC[25]. Despite ongoing challenges in creating and implementing preventive vaccines for HCV, recent advancements in treatment using specific drugs have significantly lowered the morbidity and mortality associated with HCC[26-28]. The declining trends in deaths due to HCV-related HCC are likely influenced by the cumulative impact of hepatitis C viral suppression achieved through new-generation antiviral agents[29]. The most rapid decline in mortality occurred between 1999-2004. At the same time, the overall trends of death caused by HCC declined slowly, probably due to population growth and aging[30,31].
Females demonstrated a marked decrease in mortality as compared to the male cohort. This finding has been reported previously, with most studies involving Western cohorts and two studies being in Asian cohorts[32-37]. In a large multi-ethnic Asian patient cohort, it was found that females with HCC were significantly more adherent to surveillance protocols. As a result, they often presented with less advanced stages of HCC, leading to correspondingly better overall survival rates compared to males[38]. A higher proportion of females had HCC diagnosed during regular surveillance, resulting in detection at an earlier BCLC stage, with significantly smaller tumor sizes and lower incidences of portal vein tumor invasion and extrahepatic involvement. Being diagnosed earlier allows for better therapeutic options, as seen by the trend of more women receiving curative treatments[38]. Studies have hypothesized that sex hormones may play a role in the pathogenesis of HCC, suggesting a protective effect of estrogen against the development of HCC and an increased risk associated with testosterone[39,40]. The male predominance in HCC mortality is traditionally attributed to factors such as a higher prevalence of HCV infection in males and gender differences in high-risk lifestyle behaviors, including heavier alcohol consumption and smoking among males[41].
Decreasing mortality in Eastern Asia may be related to long-term vision, cost-effective interventions, and effective medical-care systems in high-risk countries (e.g., China)[42,43]. However, HCC-related mortality showed the largest increasing trend in Central Asia. Uzbekistan has high mortality due to HCC associated with the seroprevalence of HCV infections, and the transmission of HCV was standard in medical treatment and drug abusers[44]. In Europe, there is an increasing trend in mortality, as patients successfully treated for HCV exhibit high rates of drug and liver-related mortality. Notably, overall mortality rates in these patients are significantly higher than in the general population, including for those without cirrhosis at the time of successful HCV treatment[45].
Additionally, we did not observe a decreasing trend for HCC-related mortality in American countries. Data on the prevalence, incidence, and risk factors for HCC in Latin America is limited[46]. Mendez-Sanchez N. reported that in Mexico, the cause-specific mortality rate for this condition was 4.1 per 100000 in 2000, and it rose to 4.7 per 100000 by 2006[47]. The primary causes of liver cancer in Argentina or Brazil include HCV and HBV infection, alcohol abuse, cryptogenic cirrhosis, and schistosomiasis[48,49]. However, North America showed a significant uptrend in mortality, although the results of previous literature are conflicting. Ramani et al[50-52] noted a decline in the death rate from HCV-related HCC since 2011, attributing this to various factors: (1) The introduction of Direct-acting Antivirals (DAAs); (2) Enhanced screening and better access to HCC therapies; (3) Aging of the HCV-infected birth cohort alongside competing risks from other causes of death; and (4) Inherent differences in the remaining HCV patients from the birth cohort, leading to a reduced risk of developing HCV cirrhosis and HCC. However, Younossi et al[53] showed a statistically significant increase in in-hospital mortality for HCV admissions from 2005-2009.
The mortality trend decreased in South Asian countries; however, in the past twenty years, the incidence of HCC in India has increased, especially in Mumbai, Chennai, and Bangalore[54]. Despite data showing that the incidence of HCV-related HCC in Asia has decreased since 2006, the potential risk of HCV infection cannot be ignored[55]. HCV cure is associated with a decreased risk of HCC in Sub-Saharan Africa; however, even after achieving a sustained virological response, patients with cirrhosis continue to face a risk for HCC, underscoring the necessity for ongoing surveillance[56].
Prevention of HCV via vaccination is currently unfeasible due to its extreme genetic variability, the absence of small animal models, and the complexity of its glycoproteins[57]. Prevention of HCV can be achieved through measures such as screening of blood products, using disposable needles, stringent sterilization of medical instruments, and strict measures against illegal drug use. While immediate medical intervention may not be necessary for new HCV infections, the WHO strongly recommends treatments such as pan-genotypic DAAs and interferon (IFN) for chronic cases. Sofosbuvir/velpatasvir has been shown to be effective and safe in a phase III trial for chronic HCV in the Asian population, and elbasvir/grazoprevir has also been proven effective in a phase III trial among Asia-Pacific/Russian participants[2,58,59]. These new medications offer a higher rate of sustained virological response, require shorter treatment durations, and cause fewer toxic side effects. It is widely recognized that while the risk of HCC occurrence decreases, it does not completely disappear after viral eradication achieved by either DAAs or IFN therapies[60]. Developing new therapies could minimize progress from chronic HCV infection to HCC. However, patients with advanced liver fibrosis still require regular surveillance after HCV eradication. The use of DAAs for the management of HCV is relatively recent. More evidence is needed to show that effective treatment of HCV by DAAs can decrease the risk of developing HCV-related HCC. These medications can be costly; however, some DAAs are available at a much lower price in a few Asian countries than in the West[61]. Nevertheless, the cost may decrease as more pan-genotypic drugs are introduced, allowing for greater accessibility and a subsequent decrease in HCV-related mortality. One meta-analysis indicated no significant increase in HCC occurrence with DAA therapy compared to IFN-based treatment (RR 0.68)[62]. Another analysis showed similar risks for HCC occurrence and recurrence with DAAs and IFN-based treatments. These findings suggest comparable HCC risks between DAA and IFN therapies[63].
While this study offers a comprehensive analysis of global mortality trends in Hepatitis C-related HCC across various demographics and regions, it has several limitations. The study relies on data from the GBD database, which may have inherent biases or inaccuracies. The study does not account for the impact of healthcare access, socioeconomic factors, or comorbidities on mortality rates, potentially affecting the interpretation of the results. The study focuses solely on mortality rates, not considering other important clinical outcomes such as quality of life or disease progression. The study does not explore the potential impact of new therapeutic interventions on mortality trends. Lastly, the age-standardized estimates may only partially capture the complexities of age-related risk in different populations. These limitations should be considered when interpreting the findings and should guide future research in this area.
In conclusion, we offer a comprehensive analysis of global mortality trends associated with hepatitis C-related HCC. The data reveals significant disparities in mortality rates across different regions and demographic groups, emphasizing the critical need for targeted interventions. Advances in hepatitis C treatment have shown promise, yet the persistently high mortality rates in certain areas and among specific populations call for a multi-faceted approach. This should encompass not only medical treatment but also broader strategies like improving healthcare access, raising public awareness, and promoting early diagnosis. The findings provide a robust foundation for future research and policy initiatives to mitigate the global impact of hepatitis C-related HCC.
This research delves into the evolving global landscape of hepatocellular carcinoma (HCC) mortality, specifically focusing on its correlation with hepatitis C virus (HCV) infection. Historically, HCV has significantly influenced the etiology of chronic liver disease and liver-related malignancies, notably HCC. The study highlights the increasing global burden of HCV-related HCC, a concerning trend noted across various regions worldwide. It emphasizes the need to understand these trends in the context of recent advancements in HCV treatment and changing demographic patterns, particularly given the significant public health implications and the challenges in meeting World Health Organization's goals for viral hepatitis elimination.
There is an urgent need to address the rising global burden of HCC secondary to HCV infection. Despite advancements in treatment, HCV remains a leading cause of HCC, with varying impacts across different regions and demographics. This study aims to identify and understand these disparities to inform targeted healthcare interventions. Addressing this issue is crucial for future research and public health policy, as it directly contributes to the World Health Organization's goal of eliminating viral hepatitis as a public health threat. Understanding the regional and demographic variations in HCC mortality rates due to HCV is essential for developing effective prevention and treatment strategies, ultimately reducing the global HCC burden.
This study's principal objective is to comprehensively analyze the trends in HCC mortality associated with HCV infection across various World Bank regions. The study aims to dissect these trends by gender and geographic location, offering insights into regional and demographic disparities. A critical goal is to identify areas with rising or declining mortality rates, which could signify the effectiveness of current interventions or indicate areas needing more focused attention. Realizing these objectives is significant for future research as it provides a detailed understanding of the global landscape of HCV-related HCC. This knowledge is crucial for guiding public health policies, designing targeted interventions for at-risk populations, and shaping future studies to reduce the global burden of HCC.
Our study utilized the Global Burden of Disease database to examine HCC mortality due to HCV, focusing on different World Bank regions. We employed age-standardized mortality rates for precise demographic comparisons, analyzing these rates with Joinpoint regression software to detect trends and changes. Additionally, we used the Empirical Quantile Confidence Interval method for reliable results, despite uncertain data distributions. Our approach stands out for its regional focus and advanced statistical techniques, offering a detailed understanding of HCC mortality trends linked to HCV.
The study identified distinct regional and gender-specific trends in HCC mortality due to HCV. Key findings include a global decline in HCC mortality, with notable regional variations and gender disparities. The impact of advanced treatments like Direct-acting Antivirals (DAAs) coincided with mortality rate declines. However, North America showed an increasing trend, highlighting the need for region-specific strategies. The study underscores the importance of targeted interventions and further research to address unresolved issues in HCC mortality trends.
This study introduces a theory that regional and demographic factors significantly impact HCC mortality rates from HCV infection. It postulates that global mortality decline in some regions is due to effective DAAs use, while increases in other areas might result from varying healthcare access, public health policies, and socio-economic conditions.
Future research from this study should focus on: (1) Investigating the causes behind regional and gender disparities in HCC mortality from HCV, considering healthcare access and socio-economic factors; (2) Assessing the long-term efficacy of DAAs in preventing HCC in chronic HCV patients; (3) Evaluating and enhancing public health strategies in regions with increasing HCC mortality; (4) Continuing comprehensive data analysis for identifying new trends; and (5) Studying the socioeconomic impact of HCV and HCC, including treatment cost-effectiveness.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Yang J, Qi JL, Wang XX, Li XH, Jin R, Liu BY, Liu HX, Rao HY. The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front Public Health. 2023;11:1041201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 2. | Hepatitis C. Accessed November 1, 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. [Cited in This Article: ] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 44513] [Article Influence: 14837.7] [Reference Citation Analysis (47)] |
| 4. | Nicolini A. [Current aspects of cooperation between pediatrician, obstetrician and surgeon in favor of the malformed newborn infant]. Minerva Pediatr. 1972;24:434-439. [PubMed] [Cited in This Article: ] |
| 5. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3846] [Cited by in F6Publishing: 4111] [Article Influence: 241.8] [Reference Citation Analysis (2)] |
| 6. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1343] [Cited by in F6Publishing: 2489] [Article Influence: 829.7] [Reference Citation Analysis (1)] |
| 7. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 413] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 8. | Kuten A, Stein M, Steiner M, Rubinov R, Epelbaum R, Cohen Y. Whole abdominal irradiation following chemotherapy in advanced ovarian carcinoma. Int J Radiat Oncol Biol Phys. 1988;14:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-e616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1068] [Cited by in F6Publishing: 921] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 10. | Murai S. [Severe unilateral perceptive deafness. 3. Clinical observations on juvenile unilateral deafness]. Nihon Jibiinkoka Gakkai Kaiho. 1969;72:1140-1146. [PubMed] [Cited in This Article: ] |
| 11. | Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, Elshazly M, Esmat G, Guan R, Han KH, Koike K, Largen A, McCaughan G, Mogawer S, Monis A, Nawaz A, Piratvisuth T, Sanai FM, Sharara AI, Sibbel S, Sood A, Suh DJ, Wallace C, Young K, Negro F. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31 Suppl 2:61-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 12. | Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 323] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 13. | Yan M, Ha J, Aguilar M, Bhuket T, Liu B, Gish RG, Cheung R, Wong RJ. Birth cohort-specific disparities in hepatocellular carcinoma stage at diagnosis, treatment, and long-term survival. J Hepatol. 2016;64:326-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | El-Serag HB, Kramer J, Duan Z, Kanwal F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. 2014;109:1427-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149:1471-1482.e5; quiz e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 16. | Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, Osburn WO, Forman M, Thomas E, Thornton K, Wagner K, Vassilev V, Lin L, Lum PJ, Giudice LC, Stein E, Asher A, Chang S, Gorman R, Ghany MG, Liang TJ, Wierzbicki MR, Scarselli E, Nicosia A, Folgori A, Capone S, Cox AL. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N Engl J Med. 2021;384:541-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 17. | Bhadoria AS, Khwairakpam G, Grover GS, Pathak VK, Pandey P, Gupta R. Viral Hepatitis as a Public Health Concern: A Narrative Review About the Current Scenario and the Way Forward. Cureus. 2022;14:e21907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 18. | Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accessed November 1, 2023. Available from: https://www.who.int/publications-detail-redirect/9789240027077. [Cited in This Article: ] |
| 19. | Interim guidance for country validation of viral hepatitis elimination. Accessed November 1, 2023. Available from: https://www.who.int/publications-detail-redirect/9789240028395. [Cited in This Article: ] |
| 20. | Proctor DW, Goodall R, Borsky K, Salciccioli JD, Marshall DC, Shanmugarajah K, Mohamed A, Shalhoub J. Trends in the mortality, incidence and disability-adjusted life-years of appendicitis in EU15+ countries: an observational study of the Global Burden of Disease Database, 1990-2019. Int J Surg. 2023;109:2608-2613. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 21. | Zeng Z, Zhan J, Zhang K, Chen H, Cheng S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J Urol. 2022;40:755-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | du Prel JB, Hommel G, Röhrig B, Blettner M. Confidence interval or p-value?: part 4 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106:335-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Kim HJ, Luo J, Chen HS, Green D, Buckman D, Byrne J, Feuer EJ. Improved confidence interval for average annual percent change in trend analysis. Stat Med. 2017;36:3059-3074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Cox AL, El-Sayed MH, Kao JH, Lazarus JV, Lemoine M, Lok AS, Zoulim F. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17:533-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Pawlotsky JM. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 2004;12:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2711] [Cited by in F6Publishing: 2730] [Article Influence: 455.0] [Reference Citation Analysis (0)] |
| 27. | Turgeon MK, Lee RM, Gamboa AC, Yopp A, Ryon EL, Goel N, Wang A, Lee AY, Luu S, Hsu C, Silberfein E, Maithel SK, Russell MC. Impact of hepatitis C treatment on long-term outcomes for patients with hepatocellular carcinoma: a United States Safety Net Collaborative Study. HPB (Oxford). 2021;23:422-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Suda G, Sakamoto N. Recent advances in the treatment of hepatitis C virus infection for special populations and remaining problems. J Gastroenterol Hepatol. 2021;36:1152-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 30. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1188] [Cited by in F6Publishing: 1447] [Article Influence: 289.4] [Reference Citation Analysis (0)] |
| 31. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2632] [Cited by in F6Publishing: 2711] [Article Influence: 387.3] [Reference Citation Analysis (0)] |
| 32. | Lam CM, Yong JL, Chan AO, Ng KK, Poon RT, Liu CL, Lo CM, Fan ST. Better survival in female patients with hepatocellular carcinoma: oral contraceptive pills related? J Clin Gastroenterol. 2005;39:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Tangkijvanich P, Mahachai V, Suwangool P, Poovorawan Y. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:1547-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 51] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 34. | Phipps M, Livanos A, Guo A, Pomenti S, Yeh J, Dakhoul L, Burney H, Kettler C, Liu H, Miller E, Gawrieh S, deLemos A, Scanga A, Chalasani N, Wattacheril J. Gender Matters: Characteristics of Hepatocellular Carcinoma in Women From a Large, Multicenter Study in the United States. Am J Gastroenterol. 2020;115:1486-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Farinati F, Sergio A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Zoli M, Borzio F, Giannini EG, Caturelli E, Trevisani F; Italian Liver Cancer group. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2009;21:1212-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Dohmen K, Shigematsu H, Irie K, Ishibashi H. Longer survival in female than male with hepatocellular carcinoma. J Gastroenterol Hepatol. 2003;18:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Rich NE, Murphy CC, Yopp AC, Tiro J, Marrero JA, Singal AG. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;52:701-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Santora AH, Wroe WA. Anesthetic considerations in traumatic tracheobronchial rupture. South Med J. 1986;79:910-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Mucci LA, Kuper HE, Tamimi R, Lagiou P, Spanos E, Trichopoulos D. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. BJOG. 2001;108:291-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Tuo JY, Li HL, Wang J, Fang J, Tan YT, Xiang YB. Menstrual Factors, Reproductive History and Liver Cancer Risk: Findings from a Prospective Cohort Study in Chinese Women. Cancer Epidemiol Biomarkers Prev. 2022;31:2046-2053. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 41. | Jackson SS, Marks MA, Katki HA, Cook MB, Hyun N, Freedman ND, Kahle LL, Castle PE, Graubard BI, Chaturvedi AK. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer. 2022;128:3531-3540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 347] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 43. | Liu Z, Yang Q, Shi O, Ye W, Chen X, Zhang T. The epidemiology of hepatitis B and hepatitis C infections in China from 2004 to 2014: An observational population-based study. J Viral Hepat. 2018;25:1543-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Ruzibakiev R, Kato H, Ueda R, Yuldasheva N, Hegay T, Avazova D, Kurbanov F, Zalalieva M, Tuichiev L, Achundjanov B, Mizokami M. Risk factors and seroprevalence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection in uzbekistan. Intervirology. 2001;44:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Hamill V, Wong S, Benselin J, Krajden M, Hayes PC, Mutimer D, Yu A, Dillon JF, Gelson W, Velásquez García HA, Yeung A, Johnson P, Barclay ST, Alvarez M, Toyoda H, Agarwal K, Fraser A, Bartlett S, Aldersley M, Bathgate A, Binka M, Richardson P, Morling JR, Ryder SD, MacDonald D, Hutchinson S, Barnes E, Guha IN, Irving WL, Janjua NZ, Innes H. Mortality rates among patients successfully treated for hepatitis C in the era of interferon-free antivirals: population based cohort study. BMJ. 2023;382:e074001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Méndez-Sánchez N, Paraná R, Cheinquer H, Alves de Mattos A, Gadano A, Silva M, Pessôa MG, Gomes-Ferraz ML, Soza A, Mendes-Correa MC, Chávez-Tapia NC, Dagher L, Padilla M, Hernandez N, Sánchez-Avila JF, Contreras F, Moraes-Coelho HS, Parise ER, Bessone F, Uribe M; Latin American Association for the Study of the Liver. Latin American Association for the Study of the Liver recommendations on treatment of hepatitis C. Ann Hepatol. 2014;13 Suppl 2:s4-66. [PubMed] [Cited in This Article: ] |
| 47. | Méndez-Sánchez N, Villa AR, Vázquez-Elizondo G, Ponciano-Rodríguez G, Uribe M. Mortality trends for liver cancer in Mexico from 2000 to 2006. Ann Hepatol. 2008;7:226-229. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Fassio E, Míguez C, Soria S, Palazzo F, Gadano A, Adrover R, Landeira G, Fernández N, García D, Barbero R, Perelstein G, Ríos B, Isla R, Civetta E, Pérez Ravier R, Barzola S, Curciarello J, Colombato LA, Jmeniltzky A. Etiology of hepatocellular carcinoma in Argentina: results of a multicenter retrospective study. Acta Gastroenterol Latinoam. 2009;39:47-52. [PubMed] [Cited in This Article: ] |
| 49. | Fassio E, Díaz S, Santa C, Reig ME, Martínez Artola Y, Alves de Mattos A, Míguez C, Galizzi J, Zapata R, Ridruejo E, de Souza FC, Hernández N, Pinchuk L; Multicenter Group for Study of Hepatocarcinoma in Latin America; Asociación Latinoamericana para el Estudio del Hígado (ALEH). Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol. 2010;9:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Ramani A, Tapper EB, Griffin C, Shankar N, Parikh ND, Asrani SK. Hepatocellular Carcinoma-Related Mortality in the USA, 1999-2018. Dig Dis Sci. 2022;67:4100-4111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Innes H, McDonald SA, Hamill V, Yeung A, Dillon JF, Hayes PC, Went A, Fraser A, Bathgate A, Barclay ST, Janjua N, Goldberg DJ, Hutchinson SJ. Declining incidence of hepatitis C related hepatocellular carcinoma in the era of interferon-free therapies: A population-based cohort study. Liver Int. 2022;42:561-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Kim D, Li AA, Perumpail BJ, Gadiparthi C, Kim W, Cholankeril G, Glenn JS, Harrison SA, Younossi ZM, Ahmed A. Changing Trends in Etiology-Based and Ethnicity-Based Annual Mortality Rates of Cirrhosis and Hepatocellular Carcinoma in the United States. Hepatology. 2019;69:1064-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 53. | Younossi ZM, Otgonsuren M, Henry L, Arsalla Z, Stepnaova M, Mishra A, Venkatesan C, Hunt S. Inpatient resource utilization, disease severity, mortality and insurance coverage for patients hospitalized for hepatitis C virus in the United States. J Viral Hepat. 2015;22:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Liu Y, Liu L. Changes in the Epidemiology of Hepatocellular Carcinoma in Asia. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 55. | Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029-2041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 56. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 1000] [Article Influence: 200.0] [Reference Citation Analysis (1)] |
| 57. | Zingaretti C, De Francesco R, Abrignani S. Why is it so difficult to develop a hepatitis C virus preventive vaccine? Clin Microbiol Infect. 2014;20 Suppl 5:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Wei L, Lim SG, Xie Q, Văn KN, Piratvisuth T, Huang Y, Wu S, Xu M, Tang H, Cheng J, Le Manh H, Gao Y, Mou Z, Sobhonslidsuk A, Dou X, Thongsawat S, Nan Y, Tan CK, Ning Q, Tee HP, Mao Y, Stamm LM, Lu S, Dvory-Sobol H, Mo H, Brainard DM, Yang YF, Dao L, Wang GQ, Tanwandee T, Hu P, Tangkijvanich P, Zhang L, Gao ZL, Lin F, Le TTP, Shang J, Gong G, Li J, Su M, Duan Z, Mohamed R, Hou JL, Jia J. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 59. | George J, Burnevich E, Sheen IS, Heo J, Kinh NV, Tanwandee T, Cheng PN, Kim DY, Tak WY, Kizhlo S, Zhdanov K, Isakov V, Liang L, Lindore P, Ginanni J, Nguyen BY, Wahl J, Barr E, Robertson M, Ingravallo P, Talwani R; C‐CORAL Study Investigators. Elbasvir/grazoprevir in Asia-Pacific/Russian participants with chronic hepatitis C virus genotype 1, 4, or 6 infection. Hepatol Commun. 2018;2:595-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Dash S, Aydin Y, Widmer KE, Nayak L. Hepatocellular Carcinoma Mechanisms Associated with Chronic HCV Infection and the Impact of Direct-Acting Antiviral Treatment. J Hepatocell Carcinoma. 2020;7:45-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 62. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 63. | Rutledge SM, Zheng H, Li DK, Chung RT. No evidence for higher rates of hepatocellular carcinoma after direct-acting antiviral treatment: a meta-analysis. Hepatoma Res. 2019;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |