Copyright
©The Author(s) 2023.
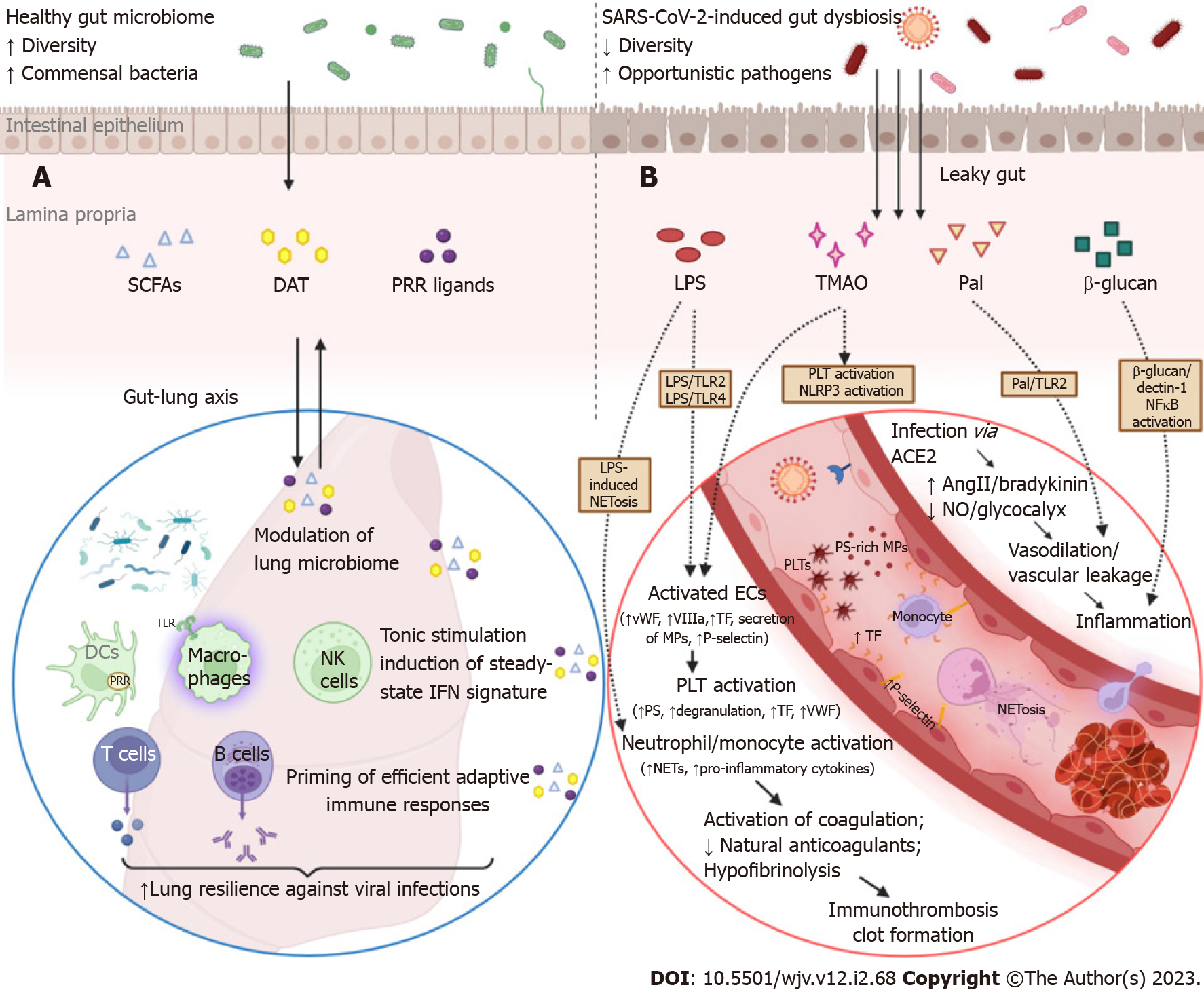
Figure 2 Overview of the sequelae of gut barrier dysfunction in severe coronavirus disease 2019: dysregulation of lung immune responses and establishment of a prothrombotic state.
A: Although the intestinal and respiratory tracts are anatomically distinct compartments, their mucosal immune cells and microbial ecosystems participate in a bidirectional immunological crosstalk (gut-lung axis). An intact intestinal barrier is pivotal in maintaining lung microbiome homeostasis and fine-tuning the respiratory immune system to elicit potent antiviral responses in the case of infection. Commensal bacteria provide tonic stimulation (through the production of pattern-recognition receptor-ligands, desaminotyrosine, short-chain fatty acids, etc.) of the epithelial, stromal, and innate immune cells of the lungs and modulate the steady-state interferon-signature, which is essential for suppressing the early phase of viral proliferation. In addition, gut-derived signals and metabolites orchestrate the effective priming of adaptive immune responses by inducing the differentiation of virus-specific CD4+ and CD8+ T cells and antibody-secreting plasma cells, which are responsible for viral control and clearance in the later stages of infection. In coronavirus disease 2019 (COVID-19), gut barrier dysfunction and depletion of symbiotic microorganisms eliminate the aforementioned immunomodulatory effects and compromise the ability of the respiratory immune system to effectively contain severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection; B: The gut vascular bed has a massive endothelial surface that is susceptible to SARS-CoV-2 infection. SARS-CoV-2 can inflict direct injury to angiotensin-converting enzyme 2 (ACE2)-expressing endothelial cells; interruption of ACE2 signaling can dysregulate both the renin-angiotensin and kinin-kallikrein systems, leading to vascular leakage. The activated or apoptotic endothelial cells release phosphatidylserine-rich endothelial microparticles and secrete large amounts of tissue factor, VIIIa, von Willebrand factor, and other procoagulant cofactors. Circulating platelets accumulate at sites of vascular injury, adhere to each other, and become activated, leading to further secretion of prothrombotic substances. Overexpression of adhesion molecules, such as P-selectin, facilitates the recruitment and activation of monocytes and other leukocytes, including neutrophil extracellular trap (NET)-producing neutrophils. COVID-19-associated immunothrombosis refers to this concurrent aberrant activation of the innate immune and coagulation systems, which predisposes to serious thrombotic complications. This vicious cycle can be further exacerbated by gut barrier dysfunction. Low-grade endotoxemia, due to increased intestinal permeability, enhances the activation of endothelial cells and platelets by inducing lipopolysaccharide (LPS)/toll-like receptor 2 (TLR2) and LPS/TRL4 downstream signaling pathways. In parallel, LPS is a potent driver of NET formation. Several other bacterial lipoproteins, such as peptidoglycan-associated lipoprotein (Pal) or Pam3Cys, aggravate vascular leakage and precipitate thrombus formation through TLR2 activation. Moreover, translocation of fungal components, such as β-glucan, could directly stimulate leukocytes and promote inflammation by binding to the Dectin-1 receptor and activating the nuclear factor-κB pathway. Finally, gut dysbiosis is associated with trimethylamine N-oxide overproduction, which is a recognized risk factor for clotting events as it enhances platelet hyperresponsiveness, endothelial dysfunction, and NLR family pyrin domain containing 3 inflammasome activation (created with biorender.com). AngII: Angiotensin II; DCs: Dendritic cells; ECs: Endothelial cells; NK cells: Natural killer cells; NO: Nitric oxide; PLT: Platelet; DAT: Desaminotyrosine; SCFAs: Short-chain fatty acids; PRRs: Pattern recognition receptors.
- Citation: Tsounis EP, Triantos C, Konstantakis C, Marangos M, Assimakopoulos SF. Intestinal barrier dysfunction as a key driver of severe COVID-19. World J Virol 2023; 12(2): 68-90
- URL: https://www.wjgnet.com/2220-3249/full/v12/i2/68.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i2.68