Copyright
©The Author(s) 2018.
World J Transplantation. Sep 10, 2018; 8(5): 122-141
Published online Sep 10, 2018. doi: 10.5500/wjt.v8.i5.122
Published online Sep 10, 2018. doi: 10.5500/wjt.v8.i5.122
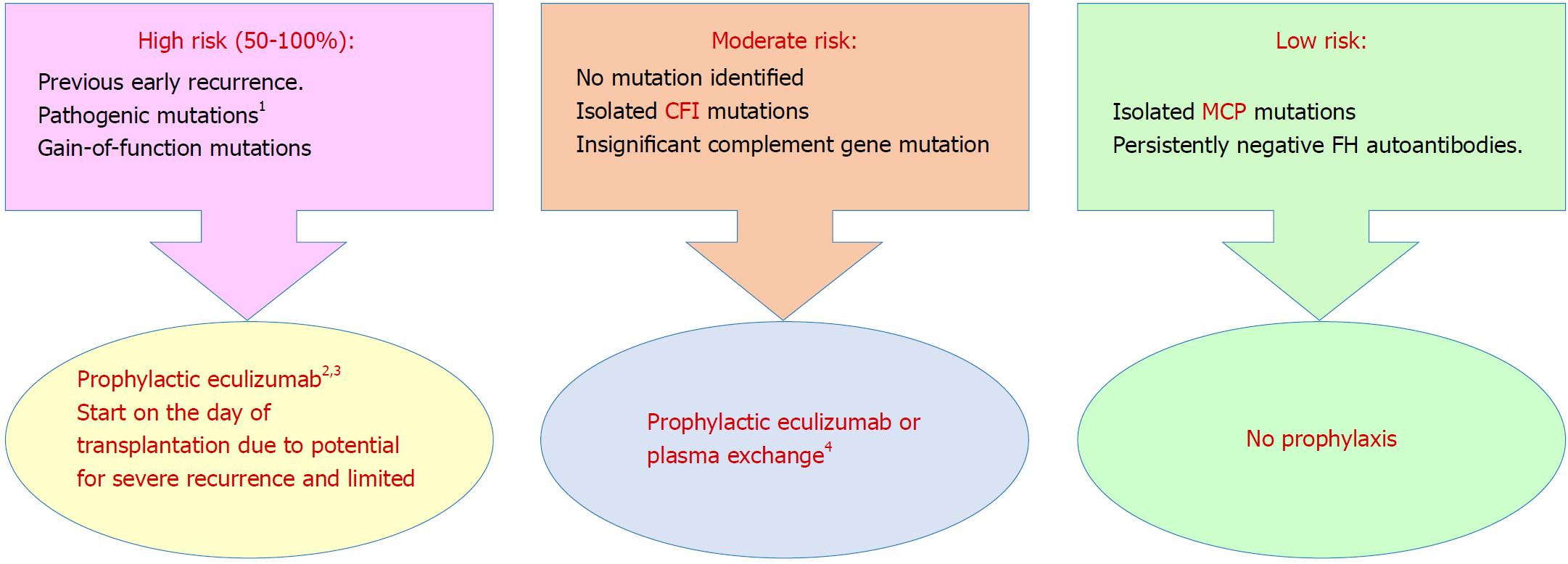
Figure 4 Prophylaxis against atypical hemolytic uremic syndrome recurrence in allograft based on a risk-assessment strategy[96] (Adapted from: Goodship et al[58]).
1Requires complete screening of all genes implicated in atypical hemolytic uremic syndrome; 2Prophylactic regimens are based on local center protocols; no trial data exist to support superiority of one protocol over another; 3Liver transplantation can be considered for renal transplant recipients with liver-derived complement protein abnormalities, uncontrolled disease activity despite eculizumab therapy or financial considerations regarding cost of long-term eculizumab therapy; 4Decision to perform or not to perform prophylactic plasma exchange or complement inhibition is left to the discretion of the clinician. aHUS: Atypical hemolytic uremic syndrome; CFI: Complement factor I gene; FH: Complement factor H protein; MCP: Membrane cofactor protein gene.
- Citation: Abbas F, El Kossi M, Kim JJ, Sharma A, Halawa A. Thrombotic microangiopathy after renal transplantation: Current insights in de novo and recurrent disease. World J Transplantation 2018; 8(5): 122-141
- URL: https://www.wjgnet.com/2220-3230/full/v8/i5/122.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i5.122