Copyright
©The Author(s) 2017.
World J Transplant. Feb 24, 2017; 7(1): 34-42
Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.34
Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.34
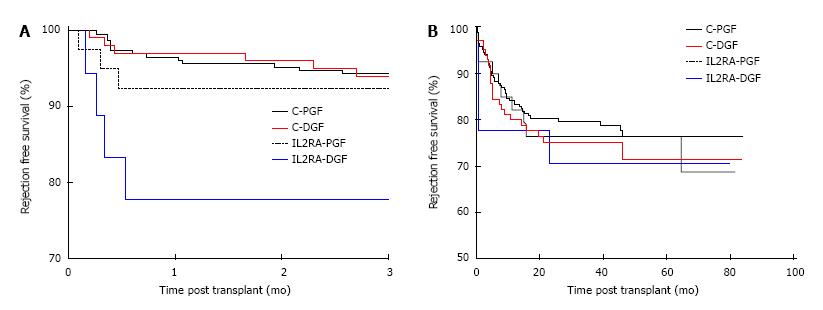
Figure 4 Three month and overall rejection free survival by induction agent and delayed graft function.
Rejection free survival, censored for DSA+ in patients with alemtuzumab induction and PGF (C-PGF), alemtuzumab induction and DGF (C-DGF), IL2RA induction and PGF (IL2RA-PGF) and IL2RA induction and DGF (IL2RA-DGF) at A: 3 mo: 93.0%, 92.9%, 92.5% and 77.8% respectively, P = 0.03 and B: 5 year: 76.4%, 71.5%, 76.5% and 70.7%, P = 0.75. DGF: Delayed graft function; PGF: Primary graft function.
- Citation: Willicombe M, Rizzello A, Goodall D, Papalois V, McLean AG, Taube D. Risk factors and outcomes of delayed graft function in renal transplant recipients receiving a steroid sparing immunosuppression protocol. World J Transplant 2017; 7(1): 34-42
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/34.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.34