Copyright
©The Author(s) 2023.
World J Transplant. Sep 18, 2023; 13(5): 239-249
Published online Sep 18, 2023. doi: 10.5500/wjt.v13.i5.239
Published online Sep 18, 2023. doi: 10.5500/wjt.v13.i5.239
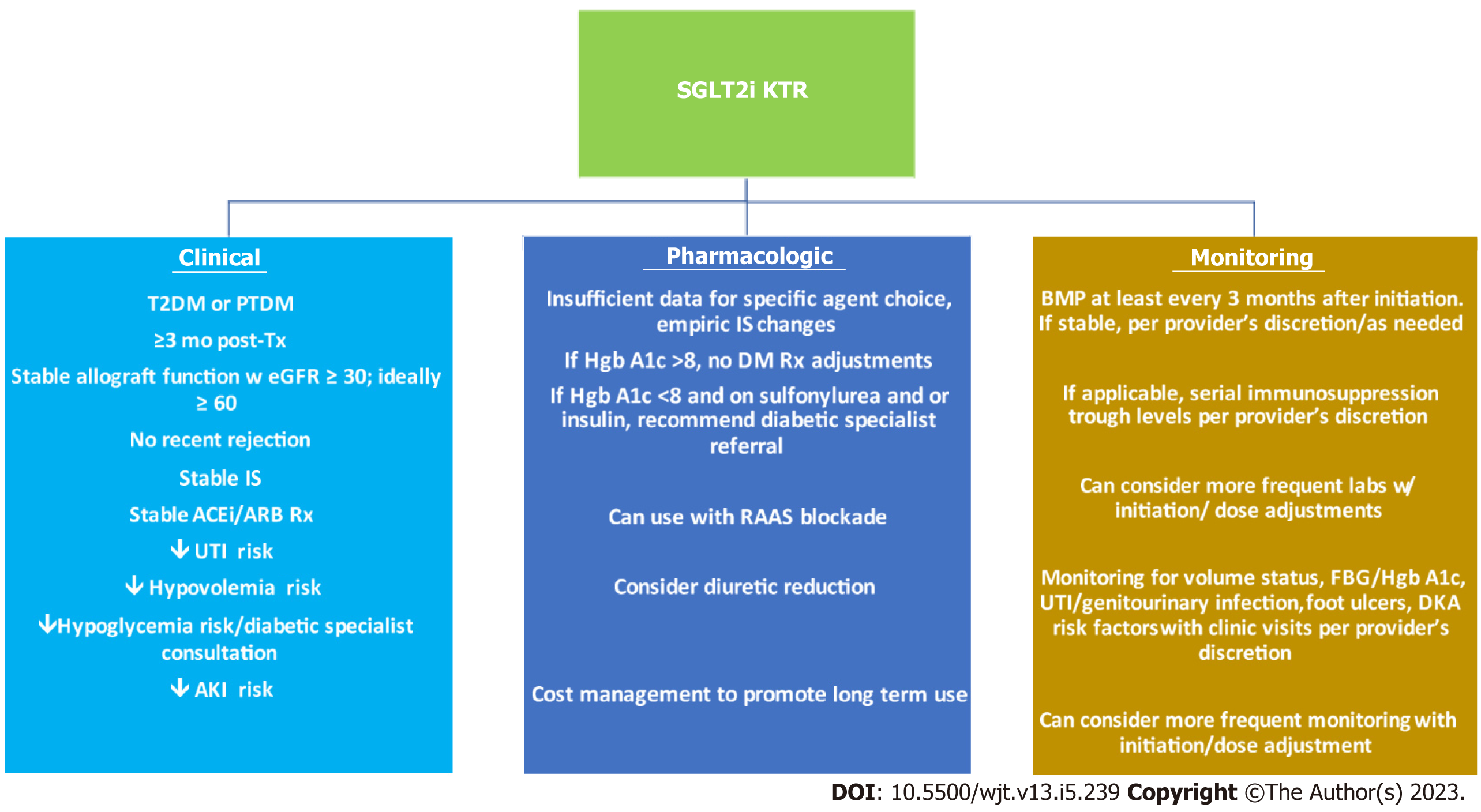
Figure 1 Clinical, pharmacologic and monitoring parameters for sodium glucose cotransporter-2 inhibitor use in a kidney transplant recipient.
Clinical criteria, pharmacologic and monitoring strategies are essential to selecting and managing kidney transplant recipient (KTRs) for sodium-glucose cotransporter-2 inhibitor (SGLT2i) use. Stable KTRs with an appropriate diabetes diagnosis, clinical stability and low risk for adverse events is key. Little data exists for empiric medication changes, though diuretic dose reduction ought to be considered. Strategies for cost management beyond insurance ought to be explored to maximize possible long-term benefit from SGLT2i administration. Laboratory and clinical monitoring more frequently with initiation/dose adjustments is critical to identifying adverse events. SGLT2i: Sodium-glucose cotransporter-2 inhibitors; KTR: Kidney transplant recipient; T2DM: Type 2 diabetes mellitus; PTDM: Post-transplant diabetes mellitus; Tx: Transplant; w: With; eGFR: Estimated glomerular filtration rate; IS: Immunosuppression; ACEi: Angiotensin converting enzyme inhibitor; ARB: Aldosterone receptor blocker; Rx: Prescription; UTI: Urinary tract infection; AKI: Acute kidney injury; Hgb: Hemoglobin; DM: Diabetes mellitus; RAAS: Renin-angiotensin-aldosterone system; FBG: Fasting blood glucose; DKA: Diabetic ketoacidosis.
- Citation: Ramakrishnan P, Garg N, Pabich S, Mandelbrot DA, Swanson KJ. Sodium-glucose cotransporter-2 inhibitor use in kidney transplant recipients. World J Transplant 2023; 13(5): 239-249
- URL: https://www.wjgnet.com/2220-3230/full/v13/i5/239.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i5.239