Copyright
©The Author(s) 2022.
World J Transplant. Aug 18, 2022; 12(8): 268-280
Published online Aug 18, 2022. doi: 10.5500/wjt.v12.i8.268
Published online Aug 18, 2022. doi: 10.5500/wjt.v12.i8.268
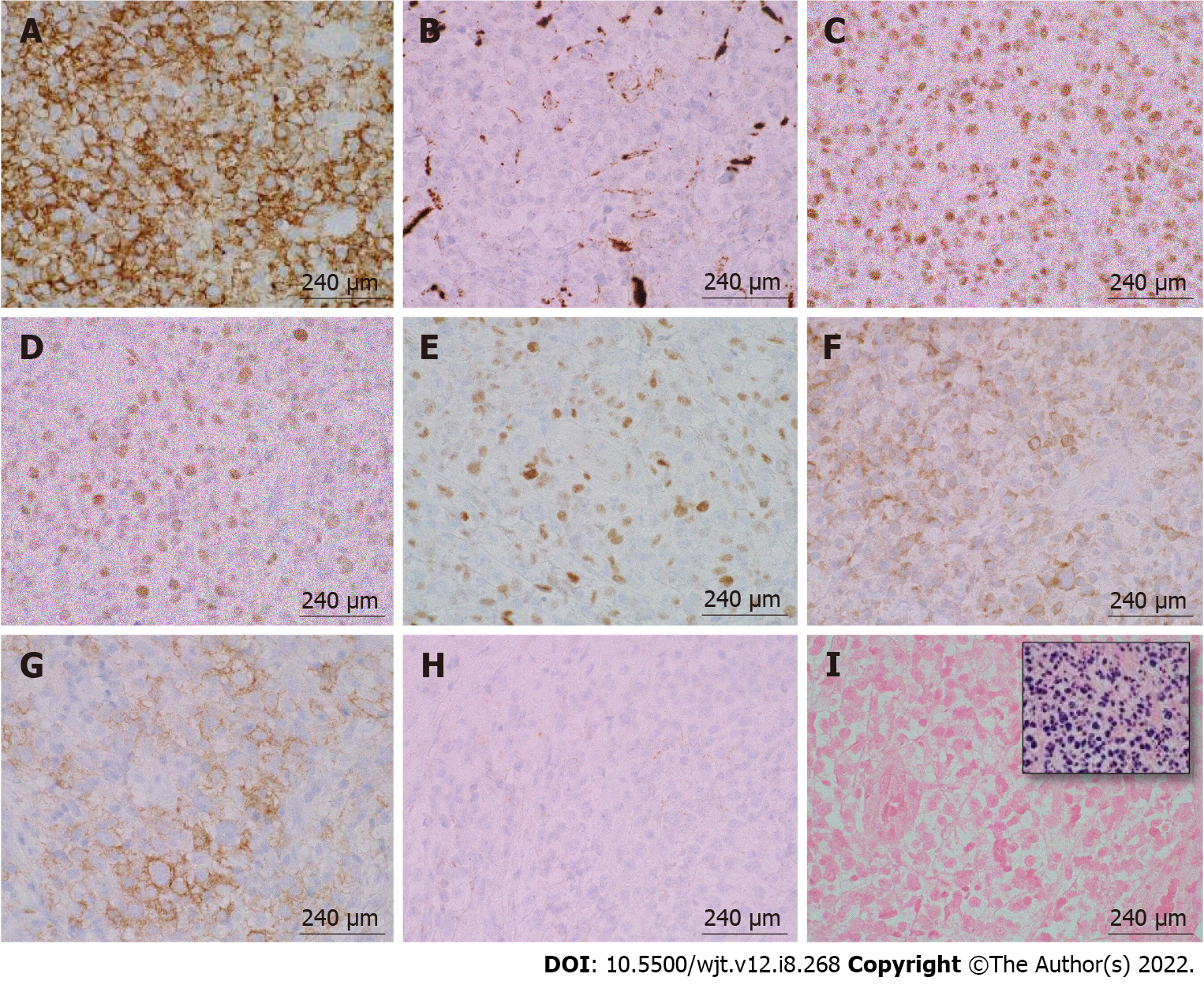
Figure 6 Immunohistochemical and in-situ hybridization staining of post-transplant lymphoproliferative disorder, monomorphic type (diffuse large B-cell lymphoma, non-germinal center type) arising from an ulcerated anal mass.
A: CD20, 100 ×/240 μm; B: CD10, 100 ×/240 μm; C: B-cell lymphoma (BCL)-6, 100 ×/240 μm; D: MUM-1, 100 ×/240 μm; E: c-MYC, 100 ×/240 μm; F: BCL-2, 100 ×/240 μm; G: CD30, 100 ×/240 μm; H: CD21, 100 ×/240 μm; I: EBER-ISH (inset: Positive control), 100 ×/240 μm. The large pleomorphic lymphocytes stained positive for CD20, B-cell lymphoma (BCL)-6, MUM-1, and BCL-2. These cells had a borderline staining for c-MYC (30%-40%), but FISH studies were negative for c-MYC rearrangements. CD30 was positive only in a subset of cells. EBER-ISH was negative.CD21 was negative and showed loss of follicular dendritic meshwork.
- Citation: Reiche W, Tauseef A, Sabri A, Mirza M, Cantu D, Silberstein P, Chandan S. Gastrointestinal manifestations, risk factors, and management in patients with post-transplant lymphoproliferative disorder: A systematic review. World J Transplant 2022; 12(8): 268-280
- URL: https://www.wjgnet.com/2220-3230/full/v12/i8/268.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i8.268