Published online Mar 18, 2022. doi: 10.5500/wjt.v12.i3.27
Peer-review started: October 22, 2021
First decision: January 22, 2022
Revised: January 27, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: March 18, 2022
Processing time: 146 Days and 16.8 Hours
Due to the optimal results obtained in kidney transplantation and to the lack of interest of the industries, new innovative drugs in kidney transplantation are difficult to be encountered. The best strategy to find the new drugs recently developed or under development is to search in the sections of kidney trans
Core Tip: Finding new innovative drugs for kidney transplantation is not easy but looking for unmet needs it is possible to find new interesting drugs and opportunities to use in kidney transplantation. Many of these drugs are just at the beginning of their process toward the approval and should be careful checked until the finish of their path. Principal unmet needs are treatment and prevention of delayed graft function, improve the long-term outcomes, desensitization and treatment of acute antibody-mediated rejection. Finding new drugs in these fields results extremely important to face new kind of transplantation as transplant from non-heart beating donor and transplant in ABO incompatibles pairs.
- Citation: Salvadori M, Tsalouchos A. Innovative immunosuppression in kidney transplantation: A challenge for unmet needs. World J Transplant 2022; 12(3): 27-41
- URL: https://www.wjgnet.com/2220-3230/full/v12/i3/27.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i3.27
Little progress has been made over the past decade in the development of new therapeutic measures in clinical kidney transplantation, chiefly because of a lack of interest by industries and providers and because most centers have reached optimal outcomes with the drugs used today[1]. However, a strategy may be adopted to identify new immunosuppressant drugs in kidney transplantation.
New immunosuppressant drugs may be found looking for identified unmet therapeutic needs.
These new drugs may also be adopted as new immunosuppressive treatments or new strategies for special kidney transplantation scenarios such as ABO incompatibility, non-heart-beating donor (NHBD) transplantation and transplantation from high-risk donors.
These drugs may be categorized as follows: (1) Therapy for ischemia-reperfusion injury (IRI) that results in delayed graft function (DGF); (2) Therapy to preserve optimal kidney function over the long-term; and (3) Therapy for desensitization and antibody-mediated rejection (ABMR).
DGF refers to acute kidney injury (AKI) occurring in the first week of transplantation that cannot not be ascribed to acute rejection[2].
DGF is associated with increased immune activation, complement activation and release of damage-associated molecular patterns, such as hypomethylated DNA, hyaluronic acid, heparin sulfate, fibrinogen and heat shock proteins. Consequently, nuclear factor κB is activated and induces inflammatory cytokines such as interleukin (IL)-1, IL-6, tumoral necrosis factor alpha and interferon beta[3].
Due to this complex mechanism, although several drugs to treat DGF have been tried, many of them failed to prove their effectiveness. Indeed, DGF has also been called the graveyard of drugs for transplantation.
However, new drugs have recently emerged and they are still in randomized controlled trials (RCTs) to control DGF.
Apoptosis plays an important role in shaping DGF. Indeed, the pro-apoptotic gene p53 is activated by hypoxia and induces cell cycle arrest and apoptosis[4].
QPI-1002 also known as 15 NP, is a short interfering RNA that inhibits the expression of p53. The results of a phase I/II clinical trial in kidney transplant recipients demonstrated beneficial effects on IRI/DGF in humans[5]. Additionally, two studies reported good results in mice[6,7]. However, the RCT was terminated in 2018 without positive results because of a lack of documented efficacy.
Carbon monoxide (CO) is involved in regulating endothelial cell survival and proliferation. It also plays roles in protecting against DGF through IRI, vessel relaxation and inhibition of proinflammatory responses[8-10]. The infusion of pegylated carboxyhemoglobin delivers CO to organs. CO is a very powerful anti-apoptotic substance and has anti-inflammatory effects. In animal studies, CO is extremely effective in both cold and warm ischemia.
The use of pegylated carboxyhemoglobin is currently the object of a phase 2/3 study to analyze the efficacy and safety of SANGUINATE for reducing the DGF rate in patients receiving a kidney transplant[11,12]. In a recent study by Thuillier et al[13], 3 oxygen transporters, HBOC-201, BbV and M101, were tested in organ preservation[13-15].
In DGF, relaxin (RLX) has an anti-inflammatory effect by reducing the expression of intracellular adhesion molecule 1, inducing the expression of Notch 1 in macrophages and reducing neutrophil adhesion through increased synthesis of nitric oxide[16-18]. Additionally, RLX causes vasodilatation through increased NO production and inhibition of endothelin 1 production[19]. Two studies[18,20] documented improved renal function, histologic improvement in damaged tissue after DGF, and a reduced number of apoptotic cells.
ANG-3777, formerly BB3, is a hepatocyte growth factor mimetic that binds to its transmembrane tyrosine kinase receptor, cMET[21]. In preclinical studies, ANG-3777 was renoprotective in a variety of animal models of AKI, exerting anti-inflammatory and regenerative effects and preventing tubular cell apoptosis, epithelial to mesenchymal transition and fibrosis[22,23]. In a randomized, placebo-controlled phase 2 trial on oliguric patients after kidney transplantation, patients treated with ANG-3777 had a larger increase in urine output, a greater reduction in C reactive protein and neutrophil gelatinase-associated lipocalin and a higher estimated glomerular filtration rate (eGFR)[24]. More recently, Vincenti et al[25] started the Graft Improvement Following Transplant (GIFT) trial, which is a phase 3 trial on the hepatocyte growth factor mimetic ANG-3777 in kidney transplant recipients with DGF. The aim of GIFT is to generate data to advance the treatment of DGF. In addition, the authors stress that a significant factor is that ANG-3777 may also be effective when administered after AKI-related DGF.
Complement activation plays a significant role in IRI, which causes and precedes DGF. The most studied among the complement inhibitor drugs to minimize DGF has been Mirocept (APT 070), which inhibits C3/C5 convertases and C1 esterase inhibitors.
Mirocept, still in a phase 1 trial (ISRCTN49958194)[26], is a potent membrane-localizing complement inhibitor and may be administered ex vivo to the donor kidney prior to transplantation. However, a recent dose finding study in animals[27] documented that a high dose of Mirocept might be needed to achieve adequate complement inhibition. More promising results have been obtained with C1 esterase inhibition.
This drug may also be administered as a donor pretreatment strategy in high-risk recipients (NCT02435732)[28], but the trial results are still unknown. Better results have been obtained by administering C1 esterase inhibitors to recipients of kidneys from high-risk donors or in the case of donation after circulatory death (DCD)[29-31]. A recent study from Huang et al[32] studied the three-year outcomes of patients treated with C1 esterase inhibitors to avoid DGF in a randomized controlled study. The study found that the treatment was associated with a lower incidence of graft failure.
Table 1 summarizes representative drugs in the categories described above used to prevent DGF and their targets.
| Drug | Molecular target | Mechanism of action |
| 15NP or QPI-1002 | p53 | Inhibition of apoptosis |
| Pegylated carboxyhemoglobin | Cytochrome C oxidase; cytochrome P450; HMGB-1; P38 MAPK pathway | Inhibition of oxidative injury, inflammation, and apoptosis |
| Relaxin | ICAM-1; neutrophil adhesion | Vasodilatation; inhibition of apoptosis |
| ANG-3777 (BB3) | Tyrosine kinase receptor cMET | Antinflammation; inhibition of epithelial to mesenchymal transition |
| Mirocept (APT 070) | Inhibition of C3/C5 convertase | Inhibition of complement activation |
| C1 esterase inhibitor | C1 esterase | Inhibition of complement activation |
Improving perfusion techniques is not drugs in the sense of the word but rather a different strategy to prevent IRI and DGF by improving kidney perfusion at the time of kidney transplantation.
In a recently published study, Urbanellis et al[33] documented that continuous normothermic ex vivo kidney perfusion significantly improved early kidney function compared with hypothermic anoxic machine perfusion and static cold storage (SCS) in a porcine kidney auto-transplantation model.
A more interesting study was performed by Niemann et al[34]. The authors documented that reducing the body temperature by 2 °C of the deceased donor achieved a significant reduction in DGF rates and that the effect was more significant in the extended criteria donors.
Finally, in a recent review[35], it was documented that active oxygenation during hypothermic machine perfusion is the most beneficial in cases involving the use of DCD kidneys when applied starting from kidney procurement until transplantation. Active oxygenation improves preservation and subsequent early graft function.
These drugs may be divided into the following categories: (1) Therapy to avoid nephrotoxicity, usually by elimination of calcineurin inhibitors (CNIs); (2) Therapy to control inflammation and fibrosis (principally when inflammation overlaps fibrosis); and (3) Therapy to prevent donor-specific antibodies (DSAs) and treat chronic ABMR (cABMR).
Until recently and even today, the two main strategies for a CNI-free regimen have been as follows: Mammalian target of rapamycin inhibitor-based immunosuppression; belatacept based immunosuppression.
Several studies have documented the efficacy of everolimus therapy in conjunction with low-dose CNIs[36-39]. The study by Pascual et al[36] “the Advancing renal TRANSplant eFficacy and safety Outcomes with eveRoliMus based regimen (TRANSFORM)” was a randomized open label, two-arm study with 2037 de novo kidney transplant recipients recruited in 186 centers worldwide. Everolimus efficacy was demonstrated, but the administration of low-dose tacrolimus (TAC) was needed.
The complete withdrawal of CNIs is difficult to achieve and is only appropriate for low-risk patients and donors and for living donors, and in the absence of DSAs[40].
The use of belatacept or other agents blocking the costimulatory pathways is the other method to avoid CNIs.
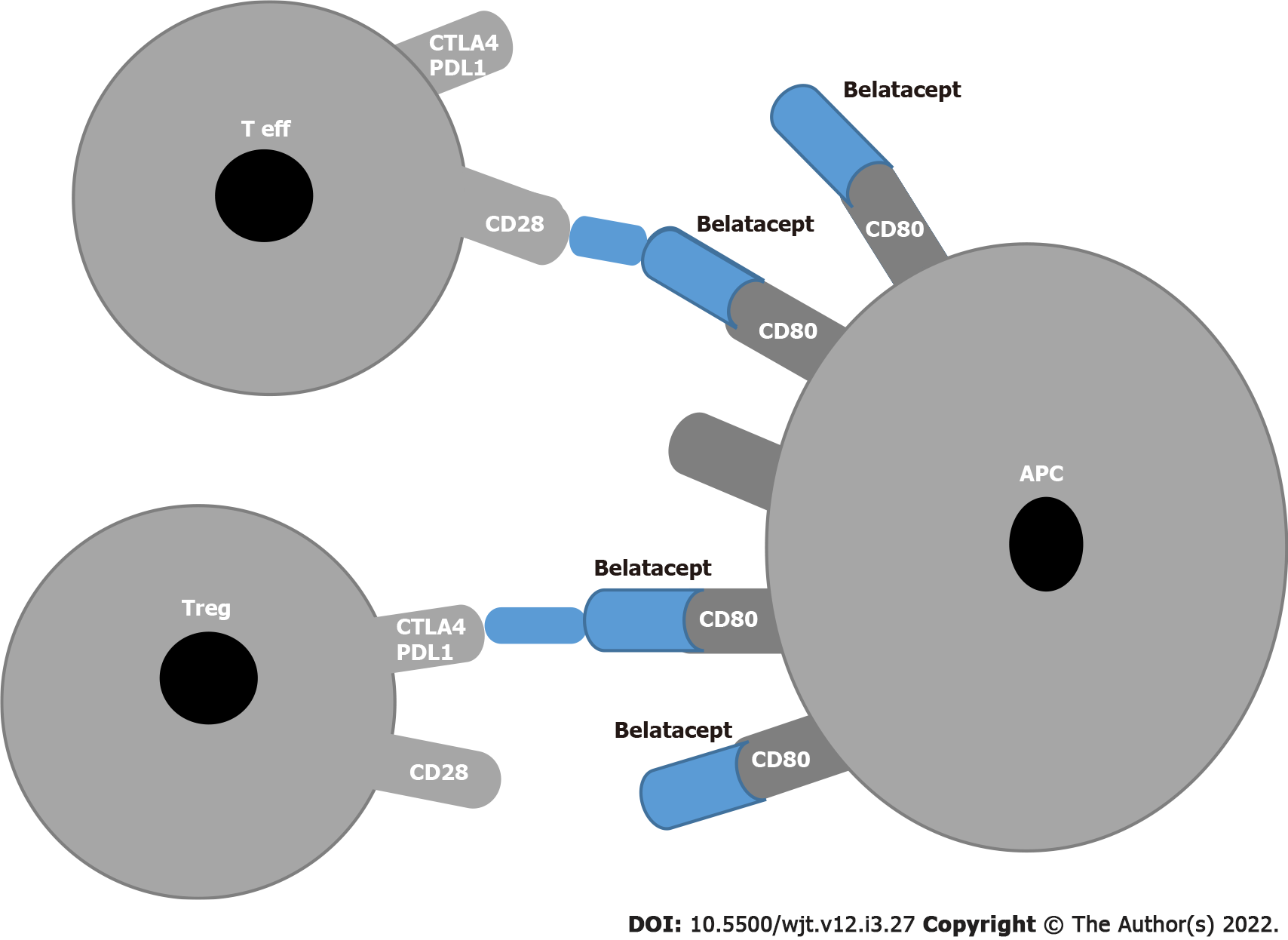
The blockade of CD28/cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) on T effector lymphocytes and CD80/CD86 on antigen presenting cells (APCs) was the first pathway to be targeted in the trials BENEFIT and BENEFIT-EXT[41,42] . Independent of well-preserved kidney function, the use of belatacept in a subset of patients was associated with an increased number of severe rejections[43,44] and an increased number of opportunistic infections[45] , including cytomegalovirus[46]. In addition a correlation between the incidence of post-lymphoproliferative disease and Epstein-Barr virus seronegative patients in the belatacept group was found[47].
These drawbacks are related to the fact that belatacept, which binds to CD80 and CD86 on APCs, blocks not only the T effectors that represent the positive signal but also the regulatory T (Tregs) that constitute the inhibitory signal (Figure 1).
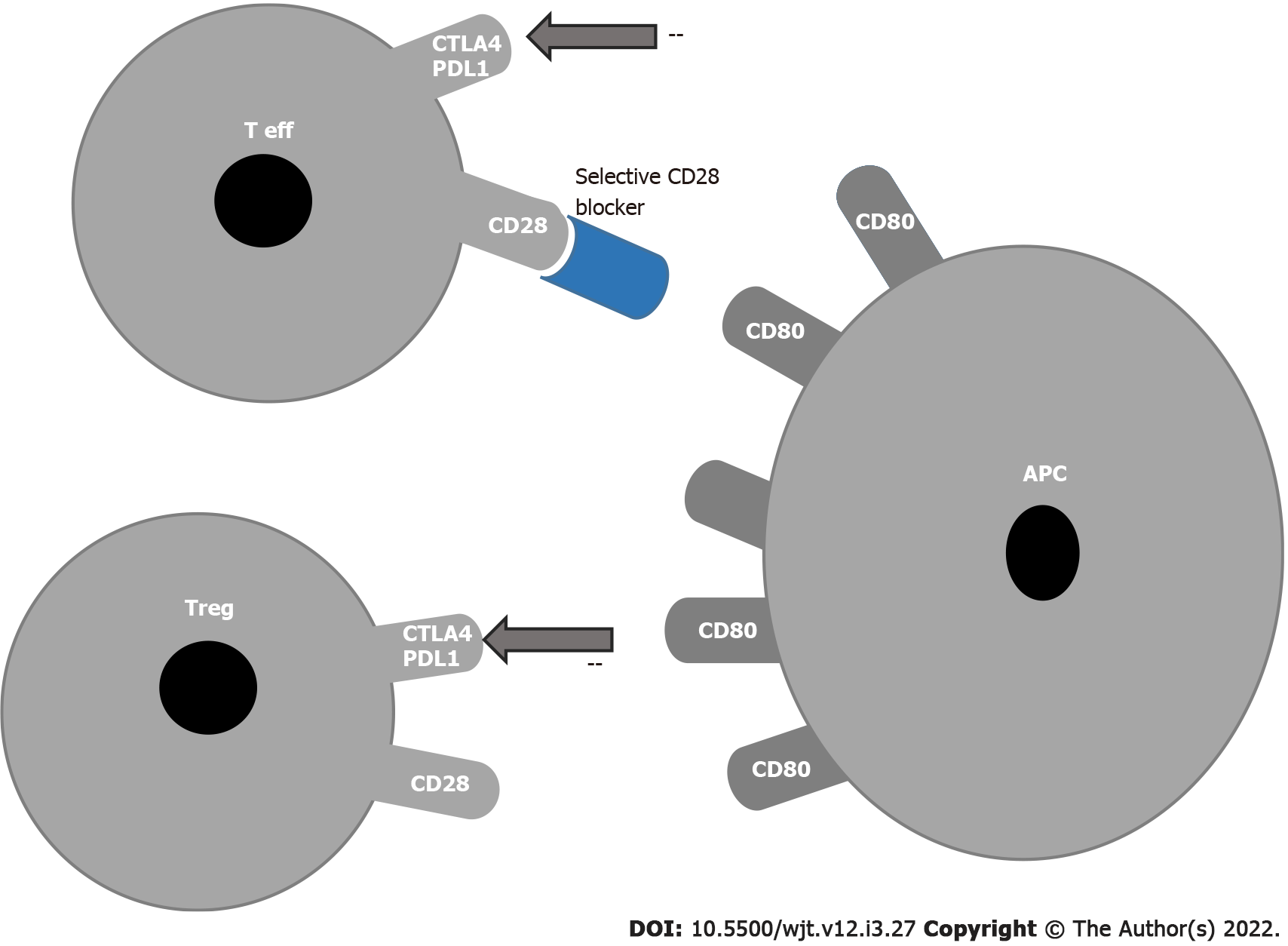
In 2015, a report showed that the blockade of CD28 on effector T cells without inhibition of Treg cells prolonged survival in a nonhuman primate kidney transplant model. In this way, effector cells can be inhibited without inhibiting Tregs because selective CD28 blockade allows inhibitory signals via CTLA-4 and programmed cell death ligand-1 to remain intact while blocking T cell activation by CD28[48] (Figure 2).
Selective targeting of the CD28 antigen on T cells might be a more effective immunosuppressive therapy than belatacept, since this blockade leaves the inhibitory signal of CTLA-4 intact and may preserve Treg functions[49-51].
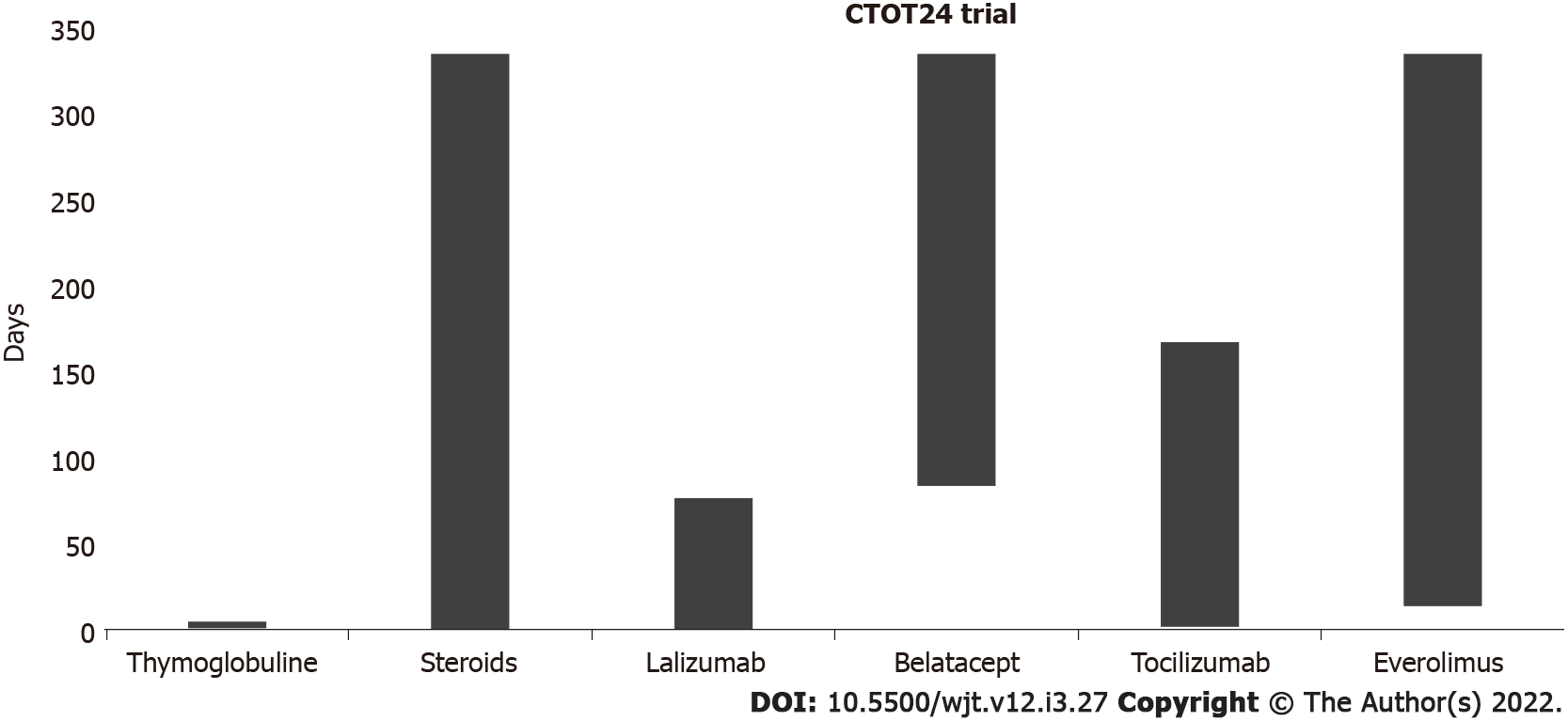
Currently, two monovalent antibodies, FR104 and lulizumab-pegol are under development for clinical application. These antibodies have antagonistic activity against CD28 alone[52,53]. To date, an RCT has been conducted at the University of California to modulate Tregs with combinatorial treatment with CD28 and IL-6 receptor antagonists[54] (Figure 3). The addition of an IL-6 receptor antagonist (tocilizumab) aims to further stimulate Treg cells and exert an anti-inflammatory effect. In the CTOT24 trial, after induction with thymoglobulin, steroids are administered from the beginning, lulizumab is started at the beginning and then continued weekly through day 77, belatacept is started on day 84 and administered every 4 wk, tocilizumab is started at the beginning and continued every 2 wk through day 168, and everolimus is started on day 14 and administered twice daily.
A different way to block costimulation is to block the interaction between CD40 and CD40 L. A first attempt was made to block the CD 40 receptor, but the studies were interrupted because of a number of thromboembolic complications[55,56]. This was because CD40 L is also expressed on platelets, which causes thromboembolic complications.
In 2014, Okimura et al[57] reported that ASKP 1240, a fully human antibody targeting human CD40, had a potent immunosuppressive effect that did not interfere with platelets.
Recently, in a phase 1b study, the safety and efficacy of bleselumab, a fully human anti-CD40 monoclonal antibody, was documented by Vincenti et al[58]. The results were confirmed by a phase 2, randomized, open label, noninferiority study by Harland et al[59].
Novartis claimed to have developed another anti-CD40 monoclonal antibody (CFZ-533, Iscalimab). The antibody was characterized by several studies[60,61] . The antibody is the object of an RCT in de novo renal transplantation[62] to demonstrate comparable efficacy to and better renal function than TAC in de novo CNI-free kidney transplantation.
Until recently, it was believed that the main cause of kidney injury over time after transplantation was primarily due to CNI nephrotoxicity.
The first study questioning this opinion was the DeKAF study by Gaston et al[63] . The study documented that the decline in kidney function was not only due to CNI nephrotoxicity but also due primarily to the presence in the recipient of DSAs and the consequent activation of the humoral response[64]. Indeed, long-term graft survival was lower in patients with DSAs in the serum and C4d, a marker of immune response activation on the glomerular capillary wall. The role of DSAs and ABMR was further documented by Sellarés et al[65] and Lefaucheur et al[66]. A separate study documented that both de novo and pre-existing DSAs caused ABMR and reduced graft survival[67].
A more recent study by Stegall et al[68] examined 575 surveillance biopsies of kidney transplants from living donors on low-dose TAC therapy and found that 82% of patients whose grafts survived 10 years were affected by inflammatory lesions not related to CNI toxicity or to immunological mechanisms.
Preserving renal function requires other therapies in addition to safely reducing or withdrawing CNIs.
Several factors, such as hyperuricemia, glucose intolerance, arterial hypertension, dyslipidemia and infection, may induce an inflammatory state in kidney transplant patients[69]. In addition, chronic hypoxia mediated by IL-1 and IL-6, angiotensin II and transforming growth factor beta may result in the accumulation of extracellular matrix, which can lead to interstitial fibrosis. In particular, several studies[70-72] document that IL-6 leads to allograft injury by acute inflammation, adaptive cellular/humoral responses, innate immunity and fibrosis. All these studies indicate that IL-6 is a mainstay in inducing inflammation and allograft injury.
Several drugs have been proposed to control the graft inflammatory state, including low-dose aspirin, statins, renin-angiotensin inhibitors, and xanthine-oxidase inhibitors, but no prospective trial with these drugs has been conducted in kidney transplantation. The only drug object of an RCT is the IL-6R inhibitor.
Currently, available agents for IL-6 signaling inhibition include monoclonal antibodies against IL-6 or IL-6R and Janus kinase inhibitors. The most often studied is tocilizumab, an IL-6R blocker. In a study conducted by Chandran et al[73], IL-6 blockade with tocilizumab increased Tregs and reduced T effector cytokines in renal graft inflammation. Tocilizumab-treated patients showed an improved tubulointerstitial Banff score and an increased Treg frequency.
Important advances have been made in the treatment of ABMR, but less effective treatments are available to control cABMR, which is a slowly progressing disease in which grafts are primarily injured by de novo DSAs[74].
Until recently, attempts to treat cABMR had been limited to a combination of plasmapheresis and intravenous immunoglobulins (IVIGs)[75] and rituximab (RTX)[76,77]. Recently, proteasome inhibitors such as bortezomib[78] and carfilzomib[79] have also been studied, but these drugs were not as effective as anticipated.
In addition, complement inhibitors such as C1 inhibitors (C1-INH) and eculizumab, failed to control cABMR[80,81] probably because antibodies may injure the endothelium in a complement-independent pathway. Better results have been obtained with the use of IL-6R or IL-6 inhibitors.
In a previous study, Shin et al[82] documented the efficacy of tocilizumab in blocking monocyte activation in an in vitro model, to inhibit the inflammatory cascade induced by alloantibodies. In a more recent study, Shin et al[83] documented a beneficial effect of tocilizumab on cABMR owing to a reduction in antibody production by B cells.
Similarly, Choi et al[84] documented a reduction in DSAs and cABMR and stabilization of renal function in patients with cABMR, DSAs and transplant glomerulopathy treated with tocilizumab. A phase 4 RCT in patients with cABMR was recently designed[85].
Clazakizumab is a humanized monoclonal antibody directed against IL-6. In a study by Dobere et al[86], clazakizumab reduced DSAs and demonstrated beneficial effects on cABMR and renal function.
Desensitization and treatment of ABMR are the two faces of the same coin. It has already been discussed how DSAs play a relevant role in inducing AKI and graft failure. DSAs may already be present before transplantation, or they may appear de novo after kidney transplantation. In both conditions, they may cause ABMR.
Desensitization is the treatment to reduce or, when possible, completely eradicate DSAs before or at the time of transplantation. Treatment of ABMR includes powerful drugs aimed at controlling this severe complication.
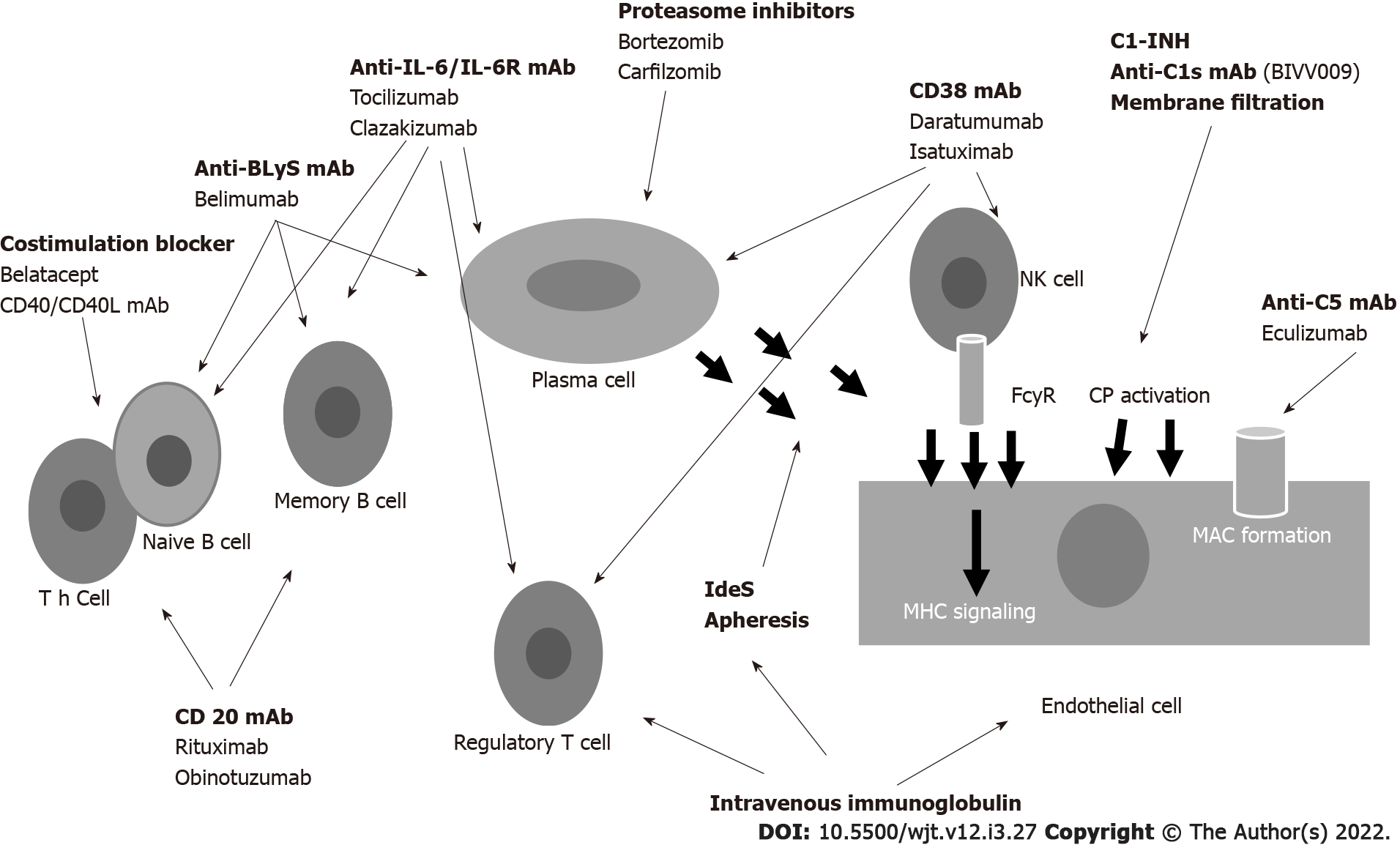
To better understand the mechanism of action of these drugs, Figure 4 represents how DSAs are formed and where the immunosuppressant drugs may act[87]. Naïve CD4+ T cells recognize the antigen presented by APCs. Activated CD4+ cells process antigens, which are presented to naïve B cells. Costimulatory molecules mediate the presentation through CD80/86 and CD28. B cell maturation and development into B-memory cells and plasma cells (PCs) is regulated by cytokines (principally IL-6 and IL-21), B cell activating factor (BAFF) and a proliferation-inducing ligand that interact with B cell maturation antigen. PCs produce antibodies that bind to donor-specific human leukocyte antigen (HLA) molecules, activate complement and initiate injury leading to ABMR. Agents capable of interfering with this complex system are numerous and act at different levels.
Several studies and reviews have described the drugs used in desensitization and in the treatment of ABMR[88-93].
Novel agents will be discussed in this chapter. New agents acting on costimulatory signals have already been discussed[48,49,57,59]. Similarly, anti-IL-6/IL-6R agents have been discussed[83-86].
Obintuzumab is a type 2 anti-CD20 antibody that induces more robust B cell depletion than RTX. To date, the drug has been evaluated in a phase 1b study to induce desensitization[94].
Belimumab belongs to the anti BAFF family. The drug is effective in treating systemic lupus erythematosus[95] but less effective in treating ABMR[96] due to possible infective complications. Proteasome inhibitors such as bortezomib and carfilzomib act on PCs, but are not as effective as anticipated. Carfilzomib has been studied in desensitization in a nonhuman primate model[97].
Drugs acting directly on PCs target CD38. Several studies or case reports have documented the efficacy of daratuzumab in the treatment of ABMR[98-100]. Isatuximab is effective on PCs and other immune cells, such as Tregs and Bregs. This fact may limit its applicability in the treatment of ABMR[101].
Inebilizumab is a humanized anti-CD19 monoclonal antibody approved for neuromyelitis optica[102].
An RCT with inebilizumab for pretransplant desensitization[103] was suspended due to the coronavirus disease pandemic.
Finally, another fully human monoclonal antibody, anti-CD38, is the object of an RCT for the treatment of ABMR[104].
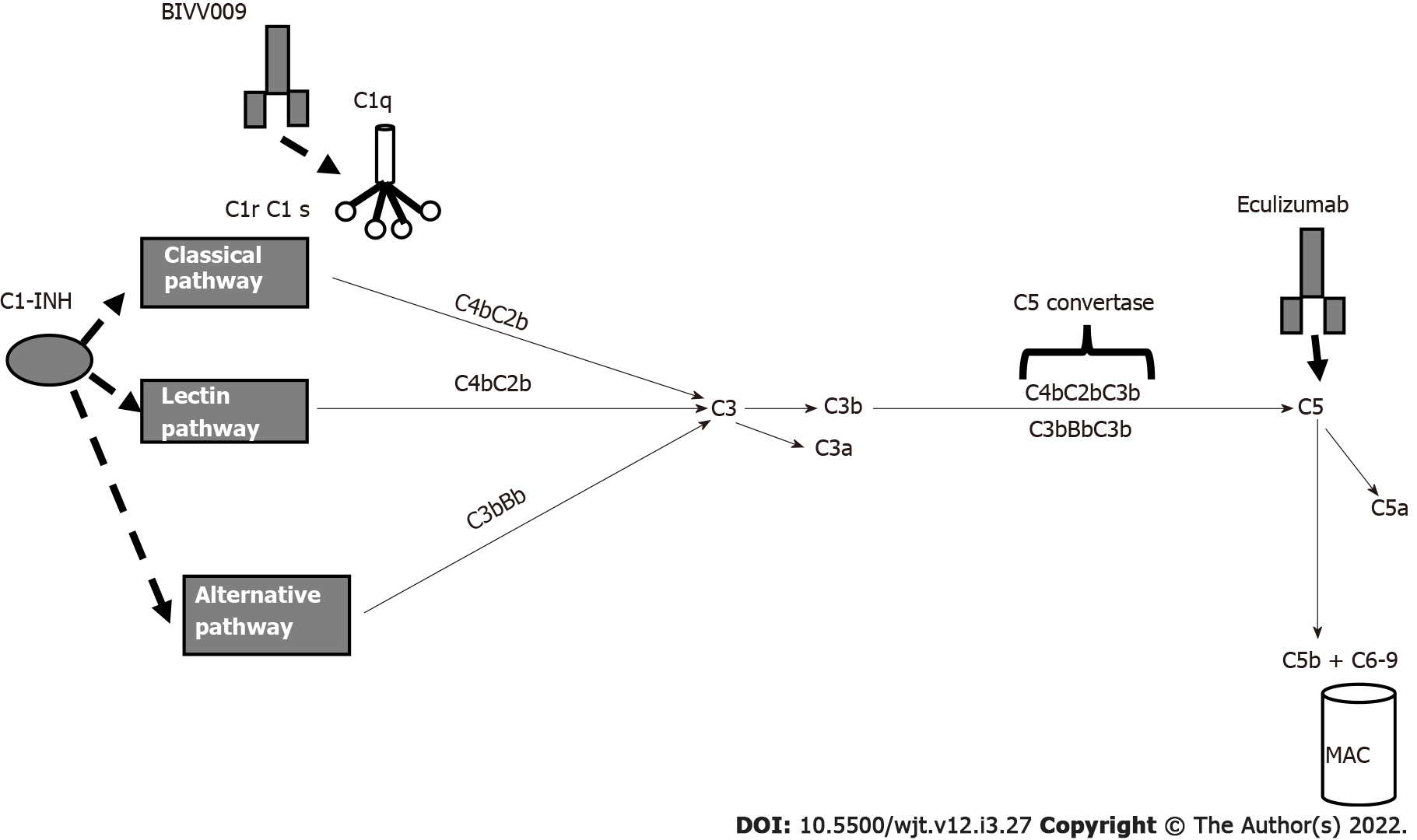
In ABMR, the activation of the complement cascade is triggered by ligation of the C1 complex to HLA antigens that are bound by DSAs. Several drugs are capable of blocking complement activation (Figure 5). The C1 complex is activated upon antibody binding. The humanized monoclonal antibody BIVV009 (sutinlimab) targets its enzymatic subcomponent C1 s and this therapy blocks C4 and C2 cleavage and the formation of C3 convertase.
A phase 1 study with this drug[105] was concluded, and Eskandary et al[80] studied 10 kidney transplant recipients with ABMR. Repeated biopsies documented a reduction in C4d deposition even if DSA levels and microvascular inflammation were unchanged.
C1-INH regulates several pathways that contribute to complement activation and cause ABMR.
In 2015, in a phase I/II placebo-controlled trial, Vo et al[106] reported the efficacy of C1-INH in the prevention of ABMR in HLA-sensitized patients. Later, Montgomery et al[107] in a randomized controlled pilot study, documented the efficacy of C1-INH in controlling ABMR. More recently, two more studies are ongoing to document the efficacy of human plasma C1 esterase inhibition as an addition to the standard of care for the treatment of ABMR[108,109].
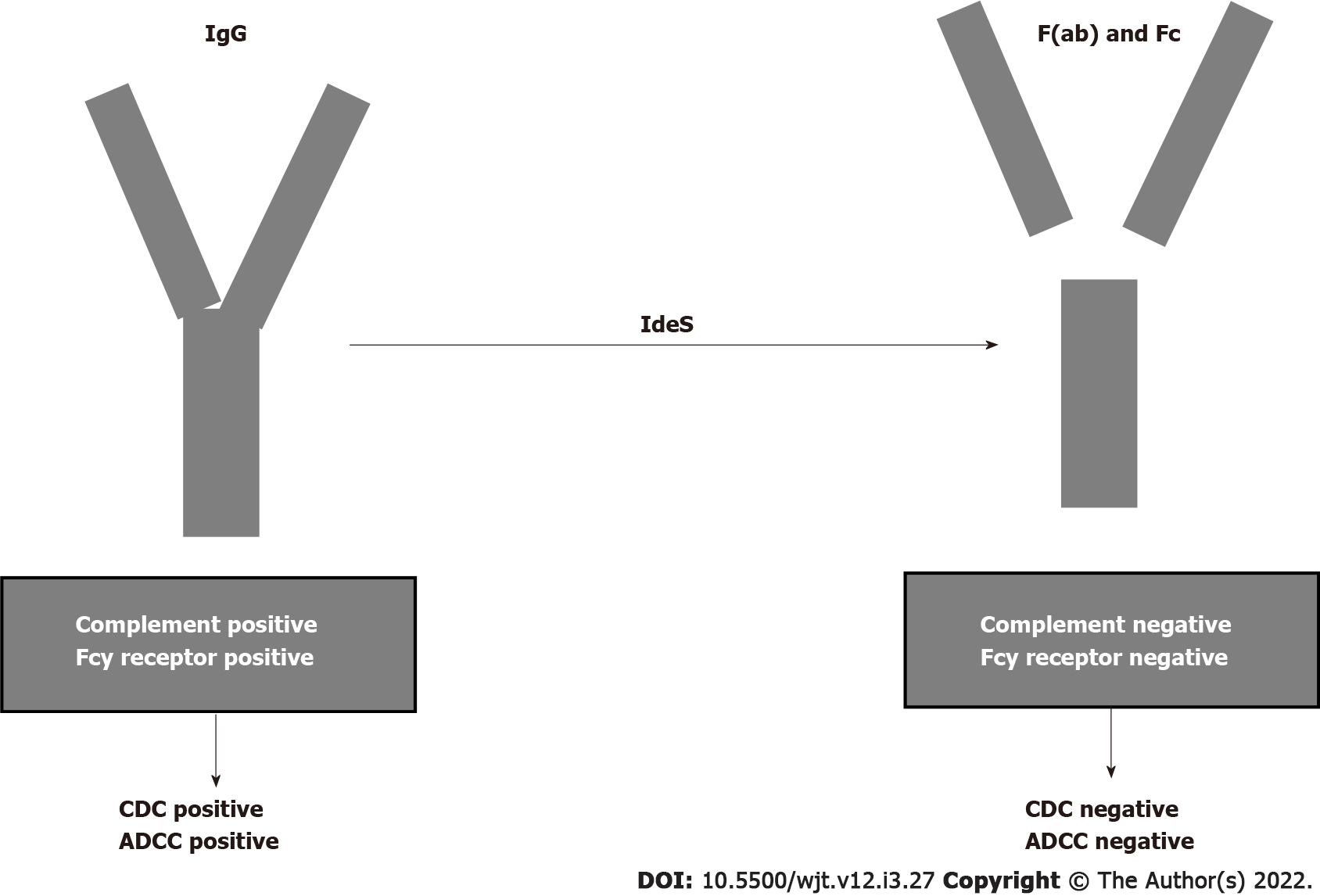
The humanized monoclonal antibody eculizumab binds to C5 with high affinity and prevents C5 convertase-mediated cleavage to C5a and C5b. In the past, several studies documented the efficacy of eculizumab in treating ABMR[110-112]. Recently, other studies documented the efficacy of eculizumab in treating and preventing ABMR[113,114]. Antibody removal is another therapeutic technique that may be applied primarily to desensitize patients with preformed DSAs before transplantation. Until recently, antibody removal and/or inhibition have been performed by plasmapheresis and IVIGs. Recently, it was documented that imlifidase (IdeS), a recombinant cysteine protease derived from Streptococcus pyogenes, rapidly cleaves IgG in the lower hinge region to a Fab fragment and a dimeric Fc fragment[115] (Figure 6). In addition to eliminating HLA antibodies, Ge et al[116] demonstrated that IdeS is a potent inhibitor of antibody-dependent cell cytotoxicity. A drawback of IdeS treatment is antibody recurrence after the interruption of the treatment. Incorporation of plasmapheresis and RTX to this treatment may overcome this drawback.
An international phase 2 trial was conducted in five transplant centers[117] for desensitization of cross-match-positive, highly sensitized kidney transplant recipients. Antibody rebound occurred 3-14 d after lipopolysaccharide administration, but graft survival at six months was 88.9%. The study conclusion was that IdeS converted positive cross matches to negative cross matches and achieved the transplantation of high-sensitized patients with optimal results at 6 mo.
In a more recent study, Kjellman et al[118] documented that lipifidase treatment administered to 39 cross-match-positive patients accomplished a 3-year graft survival of 93% with an ABMR incidence of 38% in the first month post-transplantation.
Lack of interest by industries and optimal outcomes reached by the drugs used to date has resulted in little progress in finding new drugs. However, examining unmet needs in the field of kidney transplantation may help us to find new drugs. Needs not optimally covered by current drugs are control of DGF, improvement of the long-term immunosuppression with graft outcomes reduced by chronic damage and the control of desensitization and ABMR. The control of these needs is of outmost importance, considering the expanding numbers of new kinds of kidney transplantation as transplantation from older donors and from NHBDs and transplantation from antibody-incompatible donors.
In the first kind, controlling or reducing DGF is essential; in the latter kind, the reduction of antibodies against HLA is essential.
DGF may be controlled either with optimal management of the donor before or during kidney removal or with drugs attempting to target one of the multiple pathways involved in causing the IRI that is conducive to DGF.
New drugs are also emerging to control or reduce the antibody serum level. Several steps are involved in antibody generation and for each of those steps new drugs will be found.
In addition, drugs are able to reduce the nephrotoxicity induced by the long-term use of CNIs and to control kidney inflammation that may contribute to a worse graft outcome.
The majority of these drugs have been very recently found and are still in RCTs. Therefore, trials with novel agents require a careful approach and these new agents in transplantation face many challenges, but may provide a hopeful pipeline in this issue.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaewput W, Thailand; Krishnan N, United Kingdom S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Viklicky O, Novotny M, Hruba P. Future developments in kidney transplantation. Curr Opin Organ Transplant. 2020;25:92-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279-2296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 562] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 3. | Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Moll UM, Zaika A. Nuclear and mitochondrial apoptotic pathways of p53. FEBS Lett. 2001;493:65-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Orlando G, Remuzzi G, Williams DF. Kidney Transplantation, Bioengineering and Regeneration. In: Chandran S, Vincenti F. Novel Drugs in Kidney Transplantation in Kidney Transplantation. New York: Elsevier, 2017: 277-290. [Cited in This Article: ] |
| 6. | Tchervenkov J, Squiers E, Stratta R, Odenheimer D, Rothenstein D; QPI-1002 DGF Study Group. QPI-1002, a siRNA Targeting p53: Improvement in Outcomes Following Acute Kidney Injury (AKI): Cardiac Surgery to AKI Donors. Am J Transplant. 2017;17. [Cited in This Article: ] |
| 7. | Schutt R, Case J, Barrick B, Toll A, Schaffer R, Fisher J, Marsh C. Living kidney transplant: the influence of intra-operative hemodynamics on delayed graft function. 2017 American Transplant Congress; 2017 Apr 29-May 3; Chicago, USA. [DOI] [Cited in This Article: ] |
| 8. | Ruan Y, Wang L, Zhao Y, Yao Y, Chen S, Li J, Guo H, Ming C, Gong F, Chen G. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014;86:525-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, Ikeda A, Shimizu H, Takahashi T, Tomiyama K, Sugimoto R, Choi AM, Billiar TR, Murase N, McCurry KR. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7:2279-2290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, Konduru B, Kuramitsu K, Smith NR, Berssenbrugge A, Attanasio C, Thomas M, Wegiel B, Otterbein LE. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 2010;10:2421-2430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Abuchowski A. SANGUINATE (PEGylated Carboxyhemoglobin Bovine): Mechanism of Action and Clinical Update. Artif Organs. 2017;41:346-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Pharmaceuticals P. Efficacy and Safety of SANGUINATE™ for Reduction of Delayed Graft Function in Patients Receiving a Kidney Transplant. [accessed 2015 Jul 3]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02490202 ClinicalTrials.gov Identifier: NCT02490202. [Cited in This Article: ] |
| 13. | Thuillier R, Delpy E, Matillon X, Kaminski J, Kasil A, Soussi D, Danion J, Sauvageon Y, Rod X, Donatini G, Barrou B, Badet L, Zal F, Hauet T. Preventing acute kidney injury during transplantation: the application of novel oxygen carriers. Expert Opin Investig Drugs. 2019;28:643-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Steichen C, Giraud S, Bon D, Barrou B, Badet L, Salamé E, Kerforne T, Allain G, Roumy J, Jayle C, Hannaert P, Hauet T, Thuillier R. Barriers and Advances in Kidney Preservation. Biomed Res Int. 2018;2018:9206257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Kaminski J, Hannaert P, Kasil A, Thuillier R, Leize E, Delpy E, Steichen C, Goujon JM, Zal F, Hauet T. Efficacy of the natural oxygen transporter HEMO2 life® in cold preservation in a preclinical porcine model of donation after cardiac death. Transpl Int. 2019;32:985-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Jakubauskiene L, Jakubauskas M, Leber B, Strupas K, Stiegler P, Schemmer P. Relaxin Positively Influences Ischemia-Reperfusion Injury in Solid Organ Transplantation: A Comprehensive Review. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Kageyama S, Nakamura K, Ke B, Busuttil RW, Kupiec-Weglinski JW. Serelaxin induces Notch1 signaling and alleviates hepatocellular damage in orthotopic liver transplantation. Am J Transplant. 2018;18:1755-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Alexiou K, Wilbring M, Matschke K, Dschietzig T. Relaxin protects rat lungs from ischemia-reperfusion injury via inducible NO synthase: role of ERK-1/2, PI3K, and forkhead transcription factor FKHRL1. PLoS One. 2013;8:e75592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Yoshida T, Kumagai H, Kohsaka T, Ikegaya N. Relaxin protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2013;305:F1169-F1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Collino M, Rogazzo M, Pini A, Benetti E, Rosa AC, Chiazza F, Fantozzi R, Bani D, Masini E. Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. J Cell Mol Med. 2013;17:1494-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 329] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Benoit SW, Devarajan P. Acute kidney injury: emerging pharmacotherapies in current clinical trials. Pediatr Nephrol. 2018;33:779-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Zhou D, Tan RJ, Lin L, Zhou L, Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84:509-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Bromberg JS, Weir MR, Gaber AO, Yamin MA, Goldberg ID, Mayne TJ, Cal W, Cooper M. Renal Function Improvement Following ANG-3777 Treatment in Patients at High Risk for Delayed Graft Function After Kidney Transplantation. Transplantation. 2021;105:443-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Vincenti F, Kim J, Gouveia D, Pelle G, Mayne TJ, Neylan JF. Phase 3 trial Design of the Hepatocyte Growth Factor Mimetic ANG-3777 in Renal Transplant Recipients With Delayed Graft Function. Kidney Int Rep. 2021;6:296-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Kassimatis T, Qasem A, Douiri A, Ryan EG, Rebollo-Mesa I, Nichols LL, Greenlaw R, Olsburgh J, Smith RA, Sacks SH, Drage M. A double-blind randomised controlled investigation into the efficacy of Mirococept (APT070) for preventing ischaemia reperfusion injury in the kidney allograft (EMPIRIKAL): study protocol for a randomised controlled trial. Trials. 2017;18:255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Kassimatis T, Greenlaw R, Hunter JP, Douiri A, Flach C, Rebollo-Mesa I, Nichols LL, Qasem A, Danzi G, Olsburgh J, Drage M, Friend PJ, Neri F, Karegli J, Horsfield C, Smith RA, Sacks SH. Ex vivo delivery of Mirococept: A dose-finding study in pig kidney after showing a low dose is insufficient to reduce delayed graft function in human kidney. Am J Transplant. 2021;21:1012-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | University of Winsconsin. Cinrize as a Donor Pre-treatment Strategy in Kidney Recipients of KDPI>60%. [accessed 2015 May 6]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02435732 ClinicalTrials.gov Identifier: NCT02435732. [Cited in This Article: ] |
| 29. | Jordan SC, Choi J, Aubert O, Haas M, Loupy A, Huang E, Peng A, Kim I, Louie S, Ammerman N, Najjar R, Puliyanda D, Vo A. A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Am J Transplant. 2018;18:2955-2964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Berger M, Lefaucheur C, Jordan SC. Update on C1 Esterase Inhibitor in Human Solid Organ Transplantation. Transplantation. 2019;103:1763-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Jordan S. C1INH Inhibitor Preoperative and Post Kidney Transplant to Prevent DGF & IRI (C1INHDGF). [accessed 2014 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02134314 ClinicalTrials.gov Identifier: NCT02134314. [Cited in This Article: ] |
| 32. | Huang E, Vo A, Choi J, Ammerman N, Lim K, Sethi S, Kim I, Kumar S, Najjar R, Peng A, Jordan SC. Three-Year Outcomes of a Randomized, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2020;15:109-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Urbanellis P, Hamar M, Kaths JM, Kollmann D, Linares I, Mazilescu L, Ganesh S, Wiebe A, Yip PM, John R, Konvalinka A, Mucsi I, Ghanekar A, Bagli DJ, Robinson LA, Selzner M. Normothermic Ex Vivo Kidney Perfusion Improves Early DCD Graft Function Compared With Hypothermic Machine Perfusion and Static Cold Storage. Transplantation. 2020;104:947-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, Broglio K, Hirose R, Roberts JP, Malinoski D. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med. 2015;373:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 35. | Ngo K, Castillo P, Laine RA, Sun Q. Effects of Menadione on Survival, Feeding, and Tunneling Activity of the Formosan Subterranean Termite. Insects. 2021;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y, Chadban S, Oppenheimer F, Sommerer C, Oberbauer R, Watarai Y, Legendre C, Citterio F, Henry M, Srinivas TR, Luo WL, Marti A, Bernhardt P, Vincenti F; TRANSFORM Investigators. Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation. J Am Soc Nephrol. 2018;29:1979-1991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 37. | Aala A, Brennan DC. Transformation in Immunosuppression: Are We Ready for it? J Am Soc Nephrol. 2018;29:1791-1792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Zeng J, Zhong Q, Feng X, Li L, Feng S, Fan Y, Song T, Huang Z, Wang X, Lin T. Conversion From Calcineurin Inhibitors to Mammalian Target of Rapamycin Inhibitors in Kidney Transplant Recipients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Immunol. 2021;12:663602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Montero N, Quero M, Melilli E, Pérez-Sáez MJ, Redondo-Pachón D, Bestard O, Crespo M, Cruzado JM, Pascual J. Mammalian Target of Rapamycin Inhibitors Combined With Calcineurin Inhibitors as Initial Immunosuppression in Renal Transplantation: A Meta-analysis. Transplantation. 2019;103:2031-2056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Wojciechowski D, Wiseman A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin J Am Soc Nephrol. 2021;16:1264-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 41. | Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, Garcia VD, Kamar N, Lang P, Manfro RC, Massari P, Rial MD, Schnitzler MA, Vitko S, Duan T, Block A, Harler MB, Durrbach A. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept vs cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12:630-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 42. | Rostaing L, Vincenti F, Grinyó J, Rice KM, Bresnahan B, Steinberg S, Gang S, Gaite LE, Moal MC, Mondragón-Ramirez GA, Kothari J, Pupim L, Larsen CP. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. 2013;13:2875-2883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 43. | Mathews DV, Dong Y, Higginbotham LB, Kim SC, Breeden CP, Stobert EA, Jenkins J, Tso JY, Larsen CP, Adams AB. CD122 signaling in CD8+ memory T cells drives costimulation-independent rejection. J Clin Invest. 2018;128:4557-4572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Kumar D, Raynaud M, Chang J, Reeve J, Yakubu I, Kamal L, Levy M, Bhati C, Kimball P, King A, Massey D, Halloran P, Gupta G. Impact of Belatacept Conversion on Renal Function, Histology, and Gene Expression in Kidney Transplant Patients With Chronic Active Antibody-mediated Rejection. Transplantation. 2021;105:660-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Bertrand D, Terrec F, Etienne I, Chavarot N, Sberro R, Gatault P, Garrouste C, Bouvier N, Grall-Jezequel A, Jaureguy M, Caillard S, Thervet E, Colosio C, Golbin L, Rerolle JP, Thierry A, Sayegh J, Janbon B, Malvezzi P, Jouve T, Rostaing L, Noble J. Opportunistic Infections and Efficacy Following Conversion to Belatacept-Based Therapy after Kidney Transplantation: A French Multicenter Cohort. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Chavarot N, Divard G, Scemla A, Amrouche L, Aubert O, Leruez-Ville M, Timsit MO, Tinel C, Zuber J, Legendre C, Anglicheau D, Sberro-Soussan R. Increased incidence and unusual presentations of CMV disease in kidney transplant recipients after conversion to belatacept. Am J Transplant. 2021;21:2448-2458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Ville S, Poirier N, Branchereau J, Charpy V, Pengam S, Nerriere-Daguin V, Le Bas-Bernardet S, Coulon F, Mary C, Chenouard A, Hervouet J, Minault D, Nedellec S, Renaudin K, Vanhove B, Blancho G. Anti-CD28 Antibody and Belatacept Exert Differential Effects on Mechanisms of Renal Allograft Rejection. J Am Soc Nephrol. 2016;27:3577-3588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | van der Zwan M, Hesselink DA, van den Hoogen MWF, Baan CC. Costimulation Blockade in Kidney Transplant Recipients. Drugs. 2020;80:33-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | El Hennawy H, Safar O, Al Faifi AS, El Nazer W, Kamal A, Mahedy A, Zaitoun M, Fahmy AE. Belatacept rescue therapy of CNI-induced nephrotoxicity, meta-analysis. Transplant Rev (Orlando). 2021;35:100653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int. 2011;24:2-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, Suchard SJ, Nadler SG, Ford ML. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211:297-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Shi R, Honczarenko M, Zhang S, Fleener C, Mora J, Lee SK, Wang R, Liu X, Shevell DE, Yang Z, Wang H, Murthy B. Pharmacokinetic, Pharmacodynamic, and Safety Profile of a Novel Anti-CD28 Domain Antibody Antagonist in Healthy Subjects. J Clin Pharmacol. 2017;57:161-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Poirier N, Blancho G, Hiance M, Mary C, Van Assche T, Lempoels J, Ramael S, Wang W, Thepenier V, Braudeau C, Salabert N, Josien R, Anderson I, Gourley I, Soulillou JP, Coquoz D, Vanhove B. First-in-Human Study in Healthy Subjects with FR104, a Pegylated Monoclonal Antibody Fragment Antagonist of CD28. J Immunol. 2016;197:4593-4602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | National Institute of Allergy and Infectious Diseases. Treg modulation with CD28 and IL-6 receptor antagonists. [accessed 2019 Aug 26]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04066114 ClinicalTrials.gov Identifier: NCT04066114. [Cited in This Article: ] |
| 55. | Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1492] [Cited by in F6Publishing: 1555] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 56. | Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 507] [Cited by in F6Publishing: 485] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 57. | Okimura K, Maeta K, Kobayashi N, Goto M, Kano N, Ishihara T, Ishikawa T, Tsumura H, Ueno A, Miyao Y, Sakuma S, Kinugasa F, Takahashi N, Miura T. Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects. Am J Transplant. 2014;14:1290-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Vincenti F, Klintmalm G, Yang H, Ram Peddi V, Blahunka P, Conkle A, Santos V, Holman J. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation. Am J Transplant. 2020;20:172-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Harland RC, Klintmalm G, Jensik S, Yang H, Bromberg J, Holman J, Kumar MSA, Santos V, Larson TJ, Wang X. Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am J Transplant. 2020;20:159-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 60. | Ristov J, Espie P, Ulrich P, Sickert D, Flandre T, Dimitrova M, Müller-Ristig D, Weider D, Robert G, Schmutz P, Greutmann B, Cordoba-Castro F, Schneider MA, Warncke M, Kolbinger F, Cote S, Heusser C, Bruns C, Rush JS. Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody. Am J Transplant. 2018;18:2895-2904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Espié P, He Y, Koo P, Sickert D, Dupuy C, Chokoté E, Schuler R, Mergentaler H, Ristov J, Milojevic J, Verles A, Groenewegen A, Auger A, Avrameas A, Rotte M, Colin L, Tomek CS, Hernandez-Illas M, Rush JS, Gergely P. First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody. Am J Transplant. 2020;20:463-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 62. | Novartis. CCFZ533X2201- PoC Study in de novo renal transplantation. [accessed 2014 Aug 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02217410 ClinicalTrials.gov Identifier: NCT02217410. [Cited in This Article: ] |
| 63. | Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 64. | Ye HZ, Tran HK, Van Voorhis T. Accurate Electronic Excitation Energies in Full-Valence Active Space via Bootstrap Embedding. J Chem Theory Comput. 2021;17:3335-3347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1088] [Cited by in F6Publishing: 1200] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 66. | Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Contrib Nephrol. 2009;162:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Sinha BK. Role of free radicals in etoposide (VP-16,213) action. Basic Life Sci. 1988;49:765-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 68. | Stegall MD, Cornell LD, Park WD, Smith BH, Cosio FG. Renal Allograft Histology at 10 Years After Transplantation in the Tacrolimus Era: Evidence of Pervasive Chronic Injury. Am J Transplant. 2018;18:180-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Ponticelli C, Campise MR. The inflammatory state is a risk factor for cardiovascular disease and graft fibrosis in kidney transplantation. Kidney Int. 2021;100:536-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Miller CL, Madsen JC. IL-6 Directed Therapy in Transplantation. Curr Transplant Rep. 2021;1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 71. | Jordan SC, Choi J, Kim I, Wu G, Toyoda M, Shin B, Vo A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation. 2017;101:32-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 72. | Jordan SC, Ammerman N, Choi J, Kumar S, Huang E, Toyoda M, Kim I, Wu G, Vo A. Interleukin-6: An Important Mediator of Allograft Injury. Transplantation. 2020;104:2497-2506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Chandran S, Leung J, Hu C, Laszik ZG, Tang Q, Vincenti FG. Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: A randomized controlled trial. Am J Transplant. 2021;21:2543-2554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Kim MY, Brennan DC. Therapies for Chronic Allograft Rejection. Front Pharmacol. 2021;12:651222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Redfield RR, Ellis TM, Zhong W, Scalea JR, Zens TJ, Mandelbrot D, Muth BL, Panzer S, Samaniego M, Kaufman DB, Astor BC, Djamali A. Current outcomes of chronic active antibody mediated rejection - A large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol. 2016;77:346-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Chung BH, Kim Y, Jeong HS, Hong YA, Choi BS, Park CW, Choi YJ, Kim YS, Yang CW. Clinical outcome in patients with chronic antibody-mediated rejection treated with and without rituximab and intravenous immunoglobulin combination therapy. Transpl Immunol. 2014;31:140-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Piñeiro GJ, De Sousa-Amorim E, Solé M, Ríos J, Lozano M, Cofán F, Ventura-Aguiar P, Cucchiari D, Revuelta I, Cid J, Palou E, Campistol JM, Oppenheimer F, Rovira J, Diekmann F. Rituximab, plasma exchange and immunoglobulins: an ineffective treatment for chronic active antibody-mediated rejection. BMC Nephrol. 2018;19:261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Eskandary F, Regele H, Baumann L, Bond G, Kozakowski N, Wahrmann M, Hidalgo LG, Haslacher H, Kaltenecker CC, Aretin MB, Oberbauer R, Posch M, Staudenherz A, Handisurya A, Reeve J, Halloran PF, Böhmig GA. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol. 2018;29:591-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 79. | Tremblay S, Driscoll JJ, Rike-Shields A, Hildeman DA, Alloway RR, Girnita AL, Brailey PA, Woodle ES. A prospective, iterative, adaptive trial of carfilzomib-based desensitization. Am J Transplant. 2020;20:411-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Eskandary F, Jilma B, Mühlbacher J, Wahrmann M, Regele H, Kozakowski N, Firbas C, Panicker S, Parry GC, Gilbert JC, Halloran PF, Böhmig GA. Anti-C1s monoclonal antibody BIVV009 in late antibody-mediated kidney allograft rejection-results from a first-in-patient phase 1 trial. Am J Transplant. 2018;18:916-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Schinstock CA, Bentall AJ, Smith BH, Cornell LD, Everly M, Gandhi MJ, Stegall MD. Long-term outcomes of eculizumab-treated positive crossmatch recipients: Allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am J Transplant. 2019;19:1671-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 82. | Shin BH, Ge S, Mirocha J, Jordan SC, Toyoda M. Tocilizumab (Anti-IL-6R) Suppressed TNFα Production by Human Monocytes in an In Vitro Model of Anti-HLA Antibody-Induced Antibody-Dependent Cellular Cytotoxicity. Transplant Direct. 2017;3:e139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Shin BH, Everly MJ, Zhang H, Choi J, Vo A, Zhang X, Huang E, Jordan SC, Toyoda M. Impact of Tocilizumab (Anti-IL-6R) Treatment on Immunoglobulins and Anti-HLA Antibodies in Kidney Transplant Patients With Chronic Antibody-mediated Rejection. Transplantation. 2020;104:856-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 84. | Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, Kim I, Louie S, Kang A, Peng A, Kahwaji J, Reinsmoen N, Toyoda M, Jordan SC. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am J Transplant. 2017;17:2381-2389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 85. | Baid-Agrawal S. Tocilizumab in chronic antibody-mediated rejection in kidney transplant recipients (INTERCEPT). [accessed 2020 Sep 24]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04561986 ClinicalTrials.gov Identifier: NCT04561986. [Cited in This Article: ] |
| 86. | Doberer K, Duerr M, Halloran PF, Eskandary F, Budde K, Regele H, Reeve J, Borski A, Kozakowski N, Reindl-Schwaighofer R, Waiser J, Lachmann N, Schranz S, Firbas C, Mühlbacher J, Gelbenegger G, Perkmann T, Wahrmann M, Kainz A, Ristl R, Halleck F, Bond G, Chong E, Jilma B, Böhmig GA. A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol. 2021;32:708-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 87. | Jordan SC, Ammerman N, Choi J, Huang E, Peng A, Sethi S, Najjar R, Kim I, Toyoda M, Kumar S, Lim K, Vo A. The role of novel therapeutic approaches for prevention of allosensitization and antibody-mediated rejection. Am J Transplant. 2020;20 Suppl 4:42-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Schinstock CA, Mannon RB, Budde K, Chong AS, Haas M, Knechtle S, Lefaucheur C, Montgomery RA, Nickerson P, Tullius SG, Ahn C, Askar M, Crespo M, Chadban SJ, Feng S, Jordan SC, Man K, Mengel M, Morris RE, O'Doherty I, Ozdemir BH, Seron D, Tambur AR, Tanabe K, Taupin JL, O'Connell PJ. Recommended Treatment for Antibody-mediated Rejection After Kidney Transplantation: The 2019 Expert Consensus From the Transplantion Society Working Group. Transplantation. 2020;104:911-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 89. | Nickerson PW. What have we learned about how to prevent and treat antibody-mediated rejection in kidney transplantation? Am J Transplant. 2020;20 Suppl 4:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies. J Clin Invest. 2017;127:2492-2504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 91. | Böhmig GA, Wahrmann M, Eskandary F, Rostaing L. Novel Approaches to Block Complement. Transplantation. 2018;102:1837-1843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Tatapudi VS, Montgomery RA. Therapeutic Modulation of the Complement System in Kidney Transplantation: Clinical Indications and Emerging Drug Leads. Front Immunol. 2019;10:2306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Bhalla A, Alachkar N, Alasfar S. Complement-Based Therapy in the Management of Antibody-Mediated Rejection. Adv Chronic Kidney Dis. 2020;27:138-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Redfield RR, Jordan SC, Busque S, Vincenti F, Woodle ES, Desai N, Reed EF, Tremblay S, Zachary AA, Vo AA, Formica R, Schindler T, Tran H, Looney C, Jamois C, Green C, Morimoto A, Rajwanshi R, Schroeder A, Cascino MD, Brunetta P, Borie D. Safety, pharmacokinetics, and pharmacodynamic activity of obinutuzumab, a type 2 anti-CD20 monoclonal antibody for the desensitization of candidates for renal transplant. Am J Transplant. 2019;19:3035-3045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 95. | Levy RA, Gonzalez-Rivera T, Khamashta M, Fox NL, Jones-Leone A, Rubin B, Burriss SW, Gairy K, Maurik AV, Roth DA. 10 Years of belimumab experience: What have we learnt? Lupus. 2021;30:1705-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 96. | Banham GD, Flint SM, Torpey N, Lyons PA, Shanahan DN, Gibson A, Watson CJE, O'Sullivan AM, Chadwick JA, Foster KE, Jones RB, Devey LR, Richards A, Erwig LP, Savage CO, Smith KGC, Henderson RB, Clatworthy MR. Belimumab in kidney transplantation: an experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet. 2018;391:2619-2630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 97. | Schroder PM, Schmitz R, Fitch ZW, Ezekian B, Yoon J, Choi AY, Manook M, Barbas A, Leopardi F, Song M, Farris AB, Collins B, Kwun J, Knechtle SJ. Preoperative carfilzomib and lulizumab based desensitization prolongs graft survival in a sensitized non-human primate model. Kidney Int. 2021;99:161-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | Doberer K, Kläger J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, Agis H, Reiter T, Reindl-Schwaighofer R, Wahrmann M, Cohen G, Haslacher H, Bond G, Simonitsch-Klupp I, Halloran PF, Böhmig GA. CD38 Antibody Daratumumab for the Treatment of Chronic Active Antibody-mediated Kidney Allograft Rejection. Transplantation. 2021;105:451-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 99. | Joher N, Matignon M, Grimbert P. HLA Desensitization in Solid Organ Transplantation: Anti-CD38 to Across the Immunological Barriers. Front Immunol. 2021;12:688301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 100. | Spica D, Junker T, Dickenmann M, Schaub S, Steiger J, Rüfli T, Halter J, Hopfer H, Holbro A, Hirt-Minkowski P. Daratumumab for Treatment of Antibody-Mediated Rejection after ABO-Incompatible Kidney Transplantation. Case Rep Nephrol Dial. 2019;9:149-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Feng X, Zhang L, Acharya C, An G, Wen K, Qiu L, Munshi NC, Tai YT, Anderson KC. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin Cancer Res. 2017;23:4290-4300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 102. | Frampton JE. Inebilizumab: First Approval. Drugs. 2020;80:1259-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 103. | Viela Bio. Safety and Tolerability of Inebilizumab, VIB4920, or the Combination in Highly Sensitized Candidates Awaiting Kidney Transplantation from a Deceased Donor. [accessed 2019 Nov 22]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04174677 ClinicalTrials.gov Identifier: NCT04174677. [Cited in This Article: ] |
| 104. | Eskandary F. Felzartamab in Late Antibody-Mediated Rejection (MOR202). [accessed 2021 Aug 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT05021484 ClinicalTrials.gov Identifier: NCT05021484. [Cited in This Article: ] |
| 105. | Sanofi. Safety, Tolerability and Activity of BIVV009 in Healthy Volunteers and Patients With Complement Mediated Disorders (BIVV009-01). [accessed 2015 Jul 20]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02502903 ClinicalTrials.gov Identifier: NCT02502903. [Cited in This Article: ] |
| 106. | Vo AA, Zeevi A, Choi J, Cisneros K, Toyoda M, Kahwaji J, Peng A, Villicana R, Puliyanda D, Reinsmoen N, Haas M, Jordan SC. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation. 2015;99:299-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 107. | Montgomery RA, Orandi BJ, Racusen L, Jackson AM, Garonzik-Wang JM, Shah T, Woodle ES, Sommerer C, Fitts D, Rockich K, Zhang P, Uknis ME. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody-Mediated Rejection Following Kidney Transplantation: Results of a Randomized Double-Blind Placebo-Controlled Pilot Study. Am J Transplant. 2016;16:3468-3478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 108. | CSL Behring. Efficacy and Safety of Human Plasma-derived C1-esterase Inhibitor as add-on to Standard of Care for the Treatment of Refractory Antibody Mediated Rejection (AMR) in Adult Renal Transplant Recipients. [accessed 2017 Jul 19]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03221842 ClinicalTrials.gov Identifier: NCT03221842. [Cited in This Article: ] |
| 109. | Takeda. A Multicenter Study to Evaluate the Efficacy and Safety of Cinryze® for the Treatment of Acute Antibody-mediated Rejection in Participants With Kidney Transplant. [accessed 2015 Sep 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02547220 ClinicalTrials.gov Identifier: NCT02547220. [Cited in This Article: ] |
| 110. | Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK, Stewart ZA, Warren DS, Zachary AA, Montgomery RA. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 111. | Kocak B, Arpali E, Demiralp E, Yelken B, Karatas C, Gorcin S, Gorgulu N, Uzunalan M, Turkmen A, Kalayoglu M. Eculizumab for salvage treatment of refractory antibody-mediated rejection in kidney transplant patients: case reports. Transplant Proc. 2013;45:1022-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Ghirardo G, Benetti E, Poli F, Vidal E, Della Vella M, Cozzi E, Murer L. Plasmapheresis-resistant acute humoral rejection successfully treated with anti-C5 antibody. Pediatr Transplant. 2014;18:E1-E5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Tan EK, Bentall A, Dean PG, Shaheen MF, Stegall MD, Schinstock CA. Use of Eculizumab for Active Antibody-mediated Rejection That Occurs Early Post-kidney Transplantation: A Consecutive Series of 15 Cases. Transplantation. 2019;103:2397-2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Glotz D, Russ G, Rostaing L, Legendre C, Tufveson G, Chadban S, Grinyó J, Mamode N, Rigotti P, Couzi L, Büchler M, Sandrini S, Dain B, Garfield M, Ogawa M, Richard T, Marks WH; C10-002 Study Group. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am J Transplant. 2019;19:2865-2875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, Zhang X, Eich T, Toyoda M, Eriksson BM, Ge S, Peng A, Järnum S, Wood KJ, Lundgren T, Wennberg L, Bäckman L, Larsson E, Villicana R, Kahwaji J, Louie S, Kang A, Haas M, Nast C, Vo A, Tufveson G. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N Engl J Med. 2017;377:442-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 116. | Ge S, Chu M, Choi J, Louie S, Vo A, Jordan SC, Toyoda M. Imlifidase Inhibits HLA Antibody-mediated NK Cell Activation and Antibody-dependent Cell-mediated Cytotoxicity (ADCC) In Vitro. Transplantation. 2020;104:1574-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Jordan SC, Legendre C, Desai NM, Lorant T, Bengtsson M, Lonze BE, Vo AA, Runström A, Laxmyr L, Sjöholm K, Schiött Å, Sonesson E, Wood K, Winstedt L, Kjellman C, Montgomery RA. Imlifidase Desensitization in Crossmatch-positive, Highly Sensitized Kidney Transplant Recipients: Results of an International Phase 2 Trial (Highdes). Transplantation. 2021;105:1808-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 118. | Kjellman C, Maldonado AQ, Sjöholm K, Lonze BE, Montgomery RA, Runström A, Lorant T, Desai NM, Legendre C, Lundgren T, von Zur Mühlen B, Vo AA, Olsson H, Jordan SC. Outcomes at 3 years posttransplant in imlifidase-desensitized kidney transplant patients. Am J Transplant. 2021;21:3907-3918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |