Copyright
©The Author(s) 2020.
World J Transplant. Nov 28, 2020; 10(11): 330-344
Published online Nov 28, 2020. doi: 10.5500/wjt.v10.i11.330
Published online Nov 28, 2020. doi: 10.5500/wjt.v10.i11.330
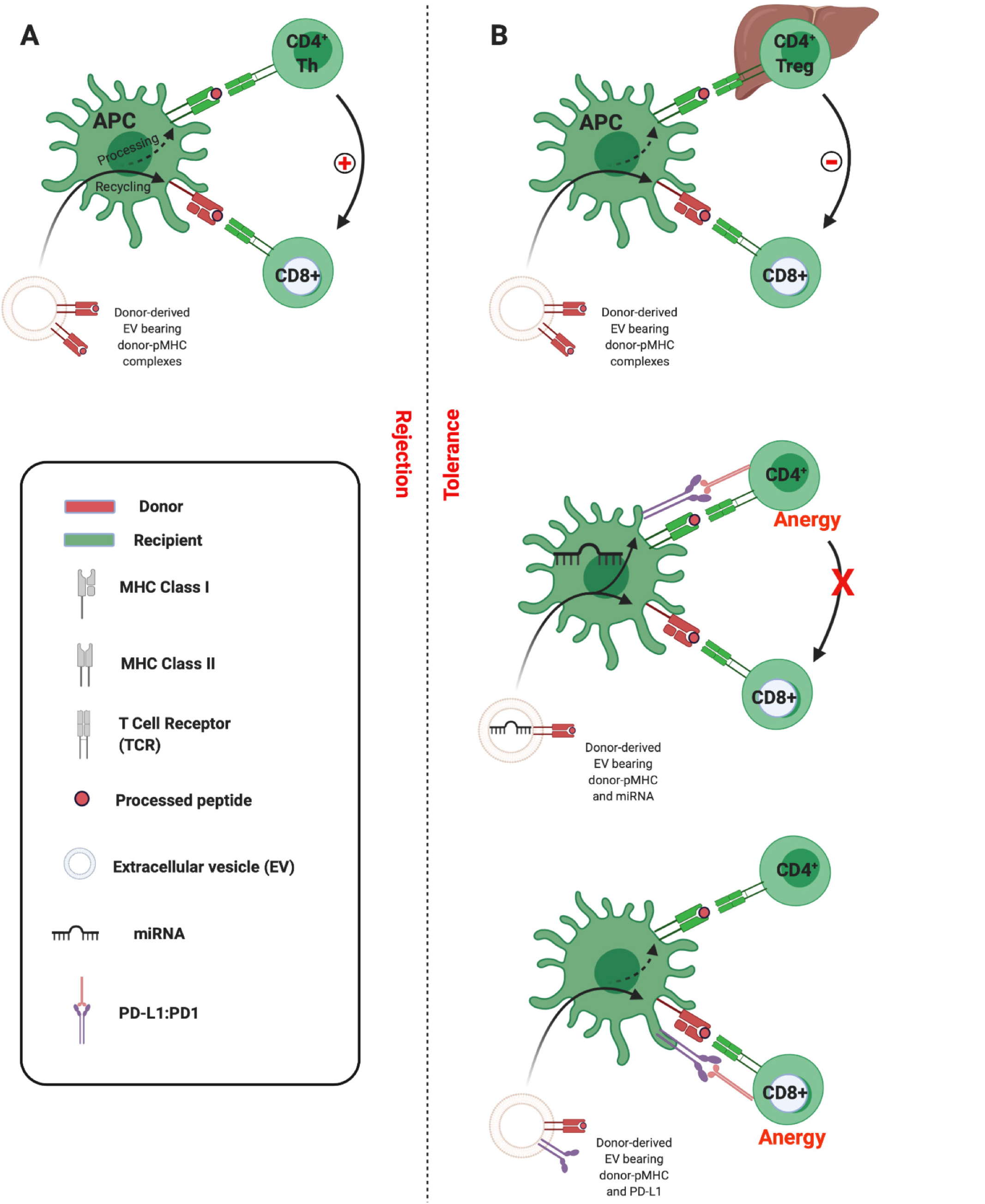
Figure 4 Three-cell model of semi-direct allorecognition.
A: Adaptive CD8 T cell immunity is the principle arm of the cellular alloimmune response, but its development requires help. This can be provided by CD4 T cells that recognise alloantigen indirectly. Extracellular vesicle (EV) cross-dressing of recipient antigen-presenting cells (APCs) can precipitate the simultaneous presentation of intact donor peptide-major histocompatibility complex (pMHC) and of processed alloantigen on self-MHC. The resultant cooperation that can occur between CD4 T cells and CD8 effector cells enables delivery of the essential help for generating the cytotoxic alloresponses forming the basis for allograft rejection; B: Under certain conditions, within the hepatic microenvironment for instance, it is possible that similar co-presentation of EV-derived alloantigen can promote CD4 regulatory T cell (Treg) suppression of effector T cells and promotion of tolerance (upper panel). Tolerance to alloantigen may also occur as a consequence of EV co-transport of nucleic acids triggering recipient APCs to upregulate immunoinhibitory molecules such as Programmed Death-Ligand 1 (middle panel), or indeed due to the tandem transfer of such intact immunoinhibitory molecules which then colocalise at the immunological synapse (lower panel). Created with BioRender.com. PD-L1: Programmed Death-Ligand 1; EV: Extracellular vesicle; APC: Antigen presenting cell; pMHC: Peptide-major histocompatibility complex.
- Citation: Mastoridis S, Martinez-Llordella M, Sanchez-Fueyo A. Extracellular vesicles as mediators of alloimmunity and their therapeutic potential in liver transplantation. World J Transplant 2020; 10(11): 330-344
- URL: https://www.wjgnet.com/2220-3230/full/v10/i11/330.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i11.330