Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.87256
Peer-review started: September 13, 2023
First decision: November 21, 2023
Revised: November 30, 2023
Accepted: December 29, 2023
Article in press: December 29, 2023
Published online: March 20, 2024
Processing time: 187 Days and 22.2 Hours
Superimposed high-frequency jet ventilation (SHFJV) is suitable for respiratory motion reduction and essential for effective lung tumor ablation. Fluid filling of the target lung wing one-lung flooding (OLF) is necessary for therapeutic ultra
To compared SHFJV with pressure-controlled ventilation (PCV) during OLF by assessing hemodynamics and gas exchange in different animal positions.
SHFJV or PCV was used alternatingly to ventilate the non-flooded lungs of the 12 anesthetized pigs during OLF. The animal positions were changed from left lateral position to supine position (SP) to right lateral position (RLP) every 30 min. In each position, ventilation was maintained for 15 min in both modalities. Hemodynamic variables and arterial blood gas levels were repeatedly measured.
Unilateral SHFJV led to lower carbon dioxide removal than PCV without ab
In porcine model, unilateral SHFJV may provide adequate ventilation in different animal positions during OLF. Lower oxygenation and CO2 removal rates compared to PCV did not lead to hypoxia or hypercapnia. SHFJV can be safely used for lung tumor ablation to minimize ventilation-induced lung motion.
Core Tip: Lung cancer prognosis is among the most unfavourable of all cancers. Therefore, there is a need to improve local lung cancer therapy while avoiding surgery. One-lung flooding (OLF) involves unilateral lung filling with saline, which generates a suitable acoustic pathway for the transthoracic application of High-intensity focused ultrasound (HIFU) in the lung. Breathing and lung movement during HIFU procedures can result in incomplete tumor ablation or collateral damage. Superimposed high-frequency jet ventilation (SHFJV) can reduce respiratory motion. However, it is unclear whether unilateral SHFJV allows adequate haemodynamics and gas exchange. In this porcine model, unilateral SHFJV may provide adequate ventilation to animals in different positions during OLF. Lower oxygenation and carbon dioxide removal rates compared to pressure controlled ventilation did not lead to hypoxia or hypercapnia. SHFJV can safely minimise ventilation-induced lung motion during lung tumor ablation.
- Citation: Lesser T, Wolfram F, Braun C, Gottschall R. Effects of unilateral superimposed high-frequency jet ventilation on porcine hemodynamics and gas exchange during one-lung flooding. World J Exp Med 2024; 14(1): 87256
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/87256.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.87256
Thermal ablation of tumors in solid organs is one of the most promising and dynamic procedures in clinical oncology[1]. However, of all the minimally invasive ablation therapies, ultrasound ablation is the only non-invasive approach to ablate the targeted tumor at depth without any needle insertion. High-intensity focused ultrasound (HIFU) is currently used to treat solid tumors, including those of the liver, breast, pancreas, kidney, and prostate[2-7]. In terms of limitations, organ movement during HIFU procedures can lead to incomplete target ablation or collateral damage[8]. Besides strategies for respiratory motion compensation such as breath hold, gating, and tumor tracking techniques[9], high-frequency jet ventilation (HFJV) utilizes very low tidal volumes, which in turn are associated with smaller respiratory movement of the lung, mediastinum and diaphragm compared with conventional ventilation[10]. Many reports describe the use of HFJV for minimally invasive treatment of tumors with different techniques and thermal ablation in the lung, liver and kidney, including percutaneous, laparoscopic, and open approaches[11,12]. However, under HFJV, a mild to moderate deterioration of gas exchange and the development of atelectasis has been observed[13,14]. Jet ventilation is the pulsed release of gas portions with high kinetic energy through adapted small-lumen tubes into the airways open to the outside. This resulted in a high gross gas volume. Simultaneous coaxial inflow and outflow are the most effective gas-transport mechanisms under jet ventilation. Because there is no gas-tight connection between the jet ventilator and the airway, intravenous anesthesia is necessary. Ventilation was indirectly measured using arterial, end-tidal, or transcutaneous CO2 measurements. The pulse frequency can be low, high, or combined superimposed HFJV (SHFJV) and is used for various clinical applications. The frequency-dependent shortening of exhalation time enables positive end-expiratory pressure (PEEP). Peak airway pressure remains comparatively low. In addition to the ventilation frequency, other adjustable jet parameters, such as jet (driving) pressure, inspiration duration, and oxygen concentration, and the individual thorax-lung mechanics, determine gas exchange[15].
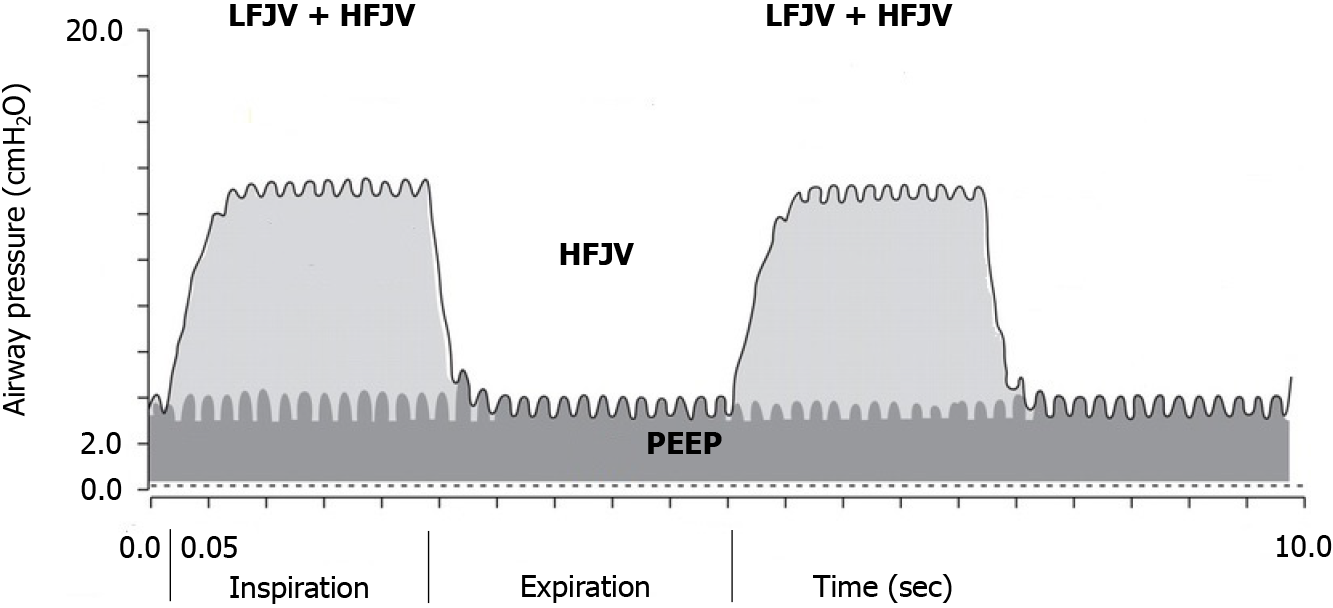
For lung tumor ablation with ultrasound, a new method for complete lung sonography has been developed[16]. This so-called one-lung flooding (OLF) involves unilateral lung filling with saline after general anesthesia and double-lumen tube intubation, which generates a suitable acoustic pathway for the transthoracic application of HIFU in the lung and liver[17,18]. Saline filling of one lung requires one-lung ventilation (OLV) of the contralateral lung. In this study, we decided to use body weight-based SHFJV for OLV. Two jet streams with different frequencies were simultaneously applied using this technique. A continuous high-frequency jet stream was superimposed during the inspiratory and expiratory phases of low-frequency jet ventilation (Figure 1).
The low-frequency jet stream resulted in phased airway pressure changes analogous to conventional ventilation with 12-20/min, providing the upper pressure level. A high-frequency jet stream with a 100-900 cycles/min frequency generates a lower pressure level (i.e., the PEEP)[19]. Compared to HFJV, SHFJV increases minute ventilation and facilitates carbon dioxide removal[20]. It may also increase end-expiratory lung volume and tidal volume, improving oxygenation[21-23].
However, studies on the unilateral SHFJV are limited. It is unclear whether unilateral SHFJV ensures adequate gas exchange during OLF. This study aimed to investigate the effects of unilateral SHFJV on hemodynamics and gas exchange during OLF compared to standard pressure-controlled ventilation (PCV). Depending on the driving pressure (DP), the low-frequency jet stream results in phased upper airway pressure changes, causing more or less large movement of the ventilated system and adjacent organs[24]. To keep the movement as low as possible, the DP of the low-frequency jet stream was chosen to be lower than the usual setting.
The patient position may be different because of the diverse forms of clinical application of HIFU treatment. Ultrasound-guided HIFU treatment requires a lateral position with the target lung above, whereas, for magnetic resonance-guided HIFU treatment, the target lung must be positioned below because the transducer is usually integrated into the magnetic resonance table. To consider different clinical applications, the effects of unilateral SHFJV during OLF on hemodynamics and gas exchange compared with PCV were investigated in different animal positions.
Seventeen juvenile female pigs (German Landrace) with a mean age of 13.6 wk (range: 12-16 wk) and a mean weight of 37.5 kg (range: 36-42 kg) were included in this study. The animals were maintained in groups and housed at the facility for four days before the study to acclimate them to their surroundings. Before the experiments, the animals were confirmed to be healthy by a veterinarian. The Veterinary Department of the Thuringian State Authority approved the present study for Food Protection and Fair Trading (TLLV Reg. 22-2684-04-WKG-16-002). All procedures were performed in the laboratories at the Central Experimental Animal Facility of Jena University Hospital in compliance with the National Animal Protection Act.
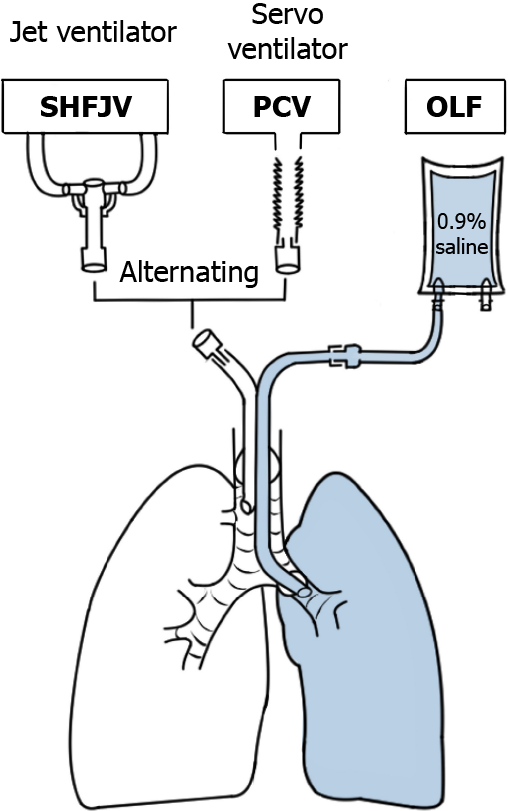
Ketamine (25 mg/kg) and midazolam (0.2 mg/kg) were administered intramuscularly as premedicants. After intravenous catheter placement in the ear vein and a bolus injection of propofol (3 mg/kg) and fentanyl (2.7 µg/kg), tracheal intubation was performed using a single-lumen endotracheal tube (6.5 mm ID; Dahlhausen, Köln, Germany). PCV was initiated (Servo 900 C; Siemens AG, Erlangen, Germany) with the following settings: fraction of inspired oxygen (FiO2), 0.4; Pinsp 15 cm H2O; inspiratory to expiratory (I:E) ratio, 1:1.9; ventilatory frequency, 20/min; and PEEP, 4 cm H2O. These settings were maintained throughout the experiment (and adjusted as necessary to maintain an end-expiratory CO2 tension of 35-45 mmHg), except for FiO2, which was increased to 1.0 for denitrogenation of the lungs before OLF. Intravenous anesthesia was initiated and maintained using a continuous intravenous infusion of propofol (6 mg/kg-1 h-1) and hourly boluses of fentanyl (2.7 µg/kg). After confirming that anesthesia was deep enough, pancuronium bromide (0.06 mg/kg hourly) maintained neuromuscular block. Electrocardiography and peripheral capillary oxygen saturation (SpO2) were monitored throughout the procedure (Datex AS/3, Datex-Ohmeda, Helsinki, Finland). Subsequently, the right common carotid artery and internal jugular vein are exposed through a cervical incision. An arterial catheter (Arterial Leader Cath, 2.7 Fr; Vygon, Ecouen, France) was placed using a sterile technique and advanced 10 cm into the central common carotid artery for invasive arterial pressure measurement and blood gas sampling. For mixed venous blood sampling and pulmonary artery pressure measurement, a flow-directed pulmonary artery catheter (6 Fr; Swan Ganz; Edwards Lifesciences, Irvine, CA, United States) was inserted through an introducer sheath (8 Fr; Arrow International, Reading, PA, United States) in the right internal jugular vein. Urinary bladder catheterization was performed. The core temperature of the animals was measured using a rectal probe. Warmed infusions and coffering of the animals using a convective warming system (WarmTouch 5800, Mallinckrodt Medical, Hennef, Germany) were used to maintain normothermia. With the pig in the supine position (SP), a 35-Fr left-sided double-lumen endobronchial tube (DLT) designed for use in pigs and specifically made for this study (Medicoplast International GmbH, Illingen, Germany)[25] was placed using an airway exchange catheter (11.0 Fr, 100 cm, extra-firm with a soft tip; COOK Deutschland GmbH, Mönchengladbach, Germany). The correct position of the DLT was confirmed using a fiber optic bronchoscope (BF 3C30; Olympus, Tokyo, Japan). A cuff controller (VBM Medizintechnik GmbH, Sulz a.N., Germany) was used to maintain a constant pressure of 50 cm H2O within both the endobronchial and tracheal cuffs. The ventilator was connected to the DLT with unchanged settings. At the end of the experiment, the animals were euthanized under deep anesthesia by injecting pentobarbital sodium.
OLF: Immediately before OLF, the animals underwent two-lung ventilation (TLV) with a FiO2 of 1.0 for 20 min to denitrogenate the lungs. The animals were then placed in the left lateral decubitus position (LLP) with the lung flooded in the dependent position, and the left (bronchial) lumen of the DLT disconnected from the ventilator. An infusion system was immediately connected to the left limb of the DLT, and the left lung was slowly filled (single filling) with degassed, warmed (37 ℃) isotonic saline flowing passively from an infusion bottle suspended 50 cm above heart level. The volume to be infused was estimated as one-half the functional residual capacity of the lungs (12.5 mL/kg)[26,27]. Complete saline filling was monitored with transcutaneous lung ultrasound (Flex Focus 800; BK Medical, Arhus, Denmark).
Before and after OLF, both lungs were conventionally ventilated using the ventilator set at the beginning of the experiment (see above). During OLF, OLV of the non-flooded right lung was performed without changing the setting.
The inspiratory hold maneuver was performed immediately before the transition from PCV to jet ventilation. A jet ventilator (TwinStreamTM ICU; Carl Reiner GmbH, Vienna, Austria) in bronchoscopy mode was connected to the right (tracheal) lumen of the DLT using a special adapter (jet converter, I.D. 15 mm; Carl Reiner GmbH, Vienna, Austria). A bias flow of warmed, humidified gas at 20 L min-1 with ventilator-identically FiO2 was connected to a jet converter (AIRcon Gen2; WILAmed GmbH, Kammerstein, Germany). The exhaled gas was hygienically filtered (DARTM Mechanical Filter Large; Covidien Ltd., Mansfield, MA, United States). The low-frequency jet stream component is primarily responsible for CO2 elimination but causes in-and expiratory movements similar to those during conventional ventilation. The extent of the movement depends on the basic/outlet DP of the low-frequency jet stream component. A previous study found that an SHFJV with a DP of 0.9 bar for the low-frequency jet stream reduces diaphragm and lung motion significantly[24]. Based on that, we found in a pilot study on three animals that this DP may ensure an acceptable gas exchange. This study aimed to verify these preliminary findings. The ventilator settings for the low-frequency jet component were 20/min, I/E = 1:2, and a basic/outlet DP of 0.9 bar. The high-frequency jet component had a 200/min frequency, an I/E ratio of 1:1, and DP of 0.4 bar. FiO2 was set at 0.4. The low- and high-frequency components were applied simultaneously (Figure 2).
Independent of the arterial partial pressure of carbon dioxide monitoring, end-expiratory CO2 tension measurements were performed every 5 min (integrated ventilator end-expiratory CO2 module). When the end-expiratory CO2 tension exceeded 45 mmHg, the DP of the low-frequency jet stream increased stepwise by 0.1 bar to a maximum of 1.3 bar.
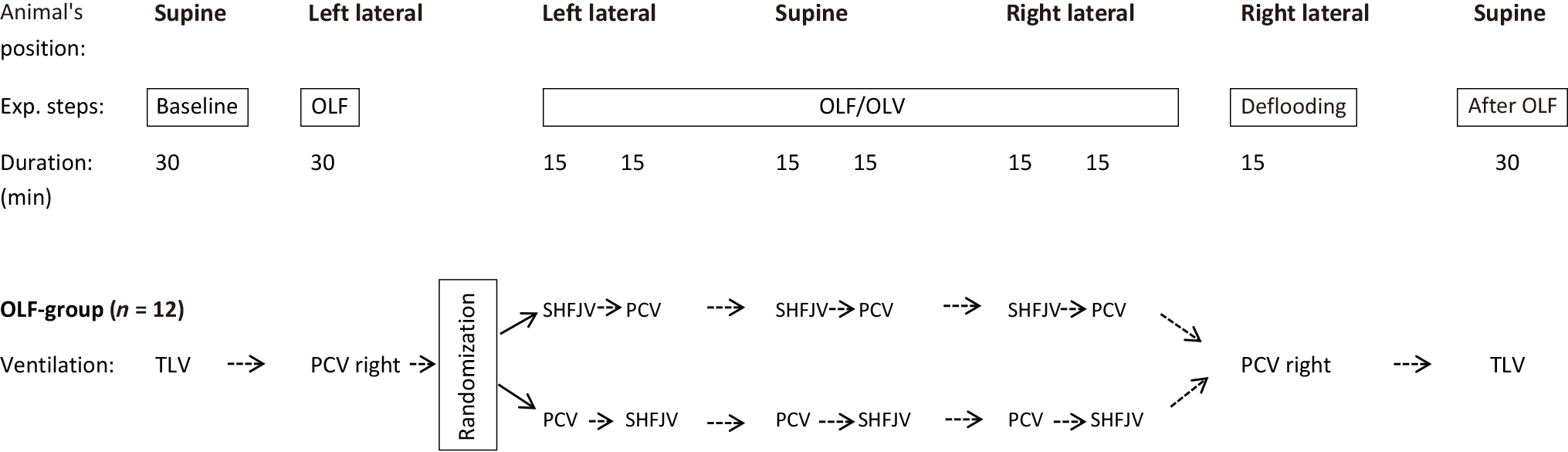
The left lung was flooded in the experimental group (OLF, n = 12), and the right lung was ventilated successively using different modes. After OLF, the animal’s position was changed every 30 min from the LLP to the SP and then to the right lateral position (RLP). Thereafter, the fluid was passively drained from the left lung, followed by conventional ventilation of both lungs (TLV) over 30 min with the animals in SP. The order in which the animals were positioned was defined as the flooding that worked best when the lung to be flooded was below it. The risk of fluid overflow in the event of tube dislocation was the greatest in the RLP; therefore, this position was used at the end of the experiment. A unilateral SHFJV or PCV was alternately used during OLF to ventilate the right lung. Ventilation modes were applied successively, and ventilation was maintained for 15 min in all modalities (Figures 3 and 4).
To minimise the influence of time point in ventilation mode on gas exchange efficacy associations with animal position, the order of the ventilation modes was randomised using a computer-generated method (Excel; Microsoft, Redmond, WA, United States). As a result, six animals underwent SHFJV or PCV at the beginning of a new body position and six each at the end of the position holding time. In the control group (n = 5), both lungs were ventilated with PCV throughout the experimental period without lung flooding; however, the position changes were the same.
Heart rate (HR); systolic, diastolic, and median arterial pressures (MAP); systolic, diastolic, and median pulmonary arterial pressure (sPAP, dPAP, mPAP); central venous pressure; and SpO2 were continuously recorded using a multiparameter patient monitor (Datex AS/3; Datex-Ohmeda, Helsinki, Finland). Arterial and mixed venous blood gases were measured at baseline, during both types of ventilation after 15 min of equilibration in each body position, and after deflooding and TLV for immediate analyses of PaO2, SaO2, arterial partial pressure of carbon dioxide (PaCO2), pH, PvO2, SvO2, PvCO2, and hemoglobin using a blood gas analyzer (Rapidpoint 405; Siemens Healthcare, Erlangen, Germany). Peak inspiratory airway pressure was measured simultaneously using a respirator. For the pulmonary right-to-left shunt fraction (Qs/Qt) calculation, the alveoloarterial oxygen difference (AaDO2) and oxygen content in the arterial and mixed venous blood (CaO2, CvO2) were used[28].
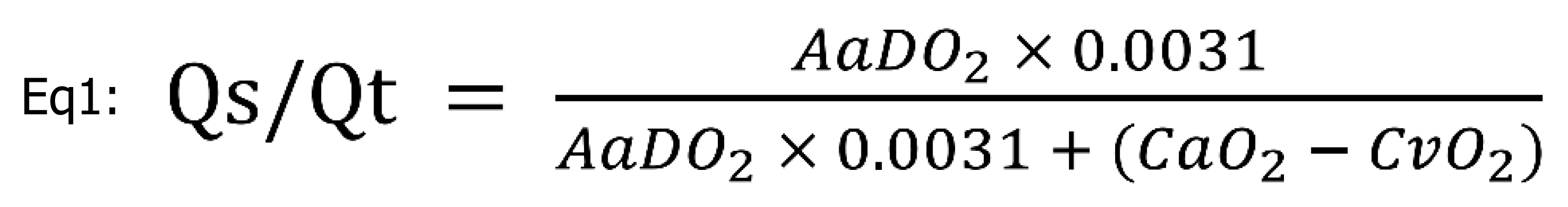
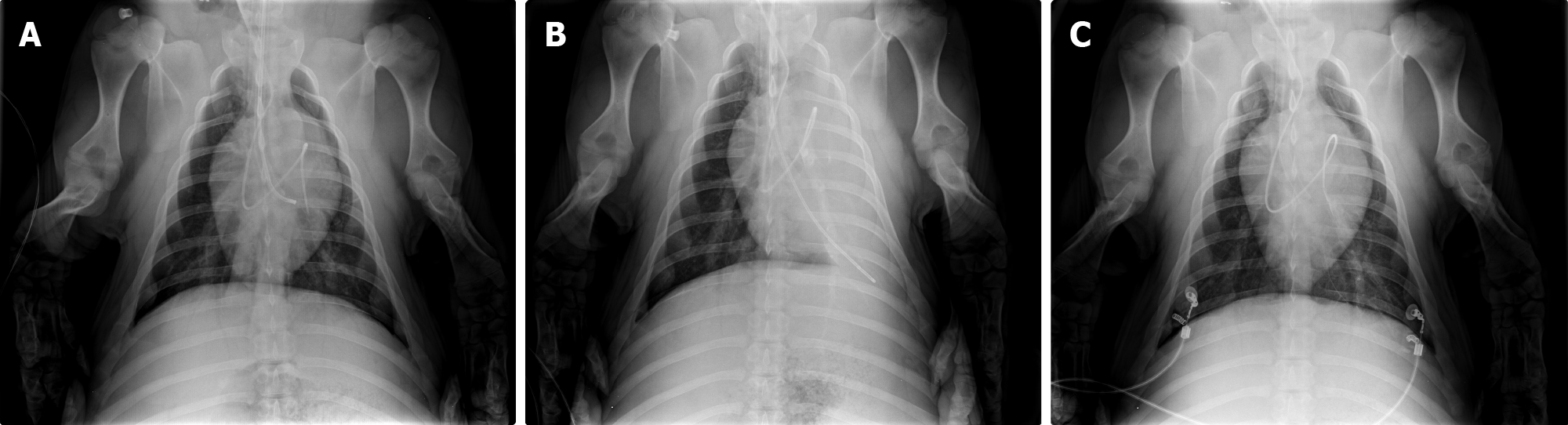
Chest X-ray was performed before, during and after OLF.
All data were analyzed using statistical software (MedCalc 19.1.7; MedCalc LTD, Ostend, Belgium) and Excel (Microsoft, Redmond, United States). Because CO2 removal is the main problem with jet ventilation, we used the PaCO2 as the primary outcome variable. Based on the assumption that a difference in PaCO2 of at least 2 kPa with a standard deviation of 0.7 kPa would be clinically relevant, we performed a power calculation (Two-Sample T-test Power Analysis). Ten animals were required for a P < 0.05, with a power of at least 80%. A total of 12 pigs were chosen to compensate for dropouts and increase the likelihood of significant findings for other important outcome variables, such as the PaO2 and shunt fraction (Qs/Qt). Statistical analysis of hemodynamic and blood gas variables was performed using the non-parametric Mann-Whitney-U test for continuous data to test the significance between independent groups (i.e., PCV vs SHFJV during OLF in each animal position). The Wilcoxon test was used between the dependent groups (baseline vs OLF/OLV and TLV), and an analysis of variance using the Friedman test was performed for analysis within the OLF/OLV groups (left vs SP vs right position). All data are presented as medians and interquartile range [Q1 Q3] (IQR). Ventilation modes were applied successively for each animal position in a randomised order. Because no differences were observed between different time points using the same ventilation mode, the collected data were merged (the Mann-Whitney-U test was used to compare data for each given ventilation mode at the beginning (n = 6) vs at the end (n = 6) of each body position). The SHFJV and PCV data reported in Tables 1 and 2 and Figures 5 and 6 are the merged data from the two-time points after 15 min of ventilation in a given ventilation mode.
| Variable | Mode | Baseline (TLV) | OLF/OLV | After OLF (TLV) | ||
| SP | LLP | SP | RLP | SP | ||
| HR (bpm) | CO-group | 98.0 (89.5-105.0) | 100.0 (98.5-104.0) | 97.0 (89.5-102.0) | 100.0 (97.0-102.5) | 97.0 (90.0-100.5) |
| PCV | 104 (98.3-109.8) | 92.0 (85.5-99.3)a | 95.0 (91.0-103.0)a | 95.0 (91.0-99.0)a | 91.0 (86.0-98.0)a | |
| SHFJVb | 91.0 (85.0-100.3) | 90.0 (85.0-95.0) | 105.0 (99.0-112.0)c | |||
| MAP (mmHg) | CO-group | 80.0 (72.5-90.5) | 78.0 (71.0-82.5) | 75.0 (67.5-82.5) | 71.0 (62.0-80.5) | 71.0 (58.0-77.0) |
| PCV | 84.5 (80.3-91.8) | 89.0 (85.3-92.8) | 80.0 (64.0-89.0) | 88.0 (68.0-101.0) | 98.0 (91.0-107.0)a | |
| SHFJV | 90.5 (88.3-94.0) | 90.0 (88.0-91.0)c | 93.0 (87.0-97.0) | |||
| sPAP (mmHg) | CO-groupb | 31 (29.5-32.0) | 29 (26.5-29.5) | 30 (29.0-33.0) | 31 (29.0-32.5) | 30 (28.0-32.0) |
| PCV | 30.5 (29.0-31.8) | 35 (32.0-38.0)a | 35 (34.0-36.0)a | 35 (33.0-40.0)a | 36 (28.0-38.0) | |
| SHFJVb | 34 (31.0-39.0) | 37 (35.0-39.0) | 43 (38.0-45.0)c | |||
| dPAP (mmHg) | CO-group | 20 (17.5-22.5) | 19 (16.5-21.5) | 20 (16.5-23.0) | 21 (19.5-23.5) | 19 (17.5-21.5) |
| PCV | 21 (19.3-22.0) | 22 (20.0-23.8) | 21 (20.0-23.0) | 21 (19.0-22.0) | 22 (19.0-24.0) | |
| SHFJVb | 20 (18.3-24.5) | 22 (21.0-23.0) | 25 (23.0-29.0)c | |||
| mPAP (mmHg) | CO-group | 24 (22.0-25.5) | 23 (20.0-25.5) | 24 (21.5-28.0) | 24 (21.5-25.0) | 22 (20.0-23.0) |
| PCV | 25 (24.0-26.0) | 28 (26.0-30.3)a | 28 (26.0-28.0)a | 28 (26.0-29.0)a | 22 (19.0-24.0)a | |
| SHFJV | 27 (25.0-30.3) | 30 (28.0-32.0) | 31 (28.0-34.0)c |
| Variable | Mode | Baseline (TLV) | OLF/OLV | after OLF (TLV) | ||
| SP | LLP | SP | RLP | SP | ||
| PaO2 (kPa) | CO-group | 20.8 (19.7-21.7) | 22.0 (21.4-25.1) | 20.5 (20.0-21.4) | 23.6 (22.0-24.1) | 22.5 (21.4- 24.3) |
| PCV | 20.6 (18.6-22.7) | 18.8 (15.3-20.4)a | 17.6 (14.7-19.1)a | 19.8 (18.5-20.3) | 22.3 (19.9-23.1) | |
| SHFJV | 14.5 (11.2-20.9) | 13.0 (12.6-15.6)c | 13.6 (11.4-17.3)c | |||
| SaO2 (%) | CO-group | 99.7 (99.7-99.8) | 99.7 (99.7-99.8) | 99.7 (99.7-99.9) | 99.7 (99.7-99.9) | 99.7 (99.7-99.8) |
| PCV | 99.7 (99.6-99.8) | 99.7 (99.1-99.9) | 99.7 (98.8-99.7) | 99.7 (99.7-99.7) | 99.7 (99.6-99.8) | |
| SHFJV | 98.3 (94.7-99.7) | 97.6 (96.4-98.7)c | 97.6 (94.399.2)c | |||
| PaCO2 (kPa) | CO-group | 5.3 (5.0-5.4) | 4.9 (4.7-5.0) | 4.8 (4.7-4.9) | 4.6 (4.54.8) | 4.6 (4.3-4.8) |
| PCV | 5.0 (4.8-6.0) | 4.7 (4.3-4.9)a | 4.7 (4.3-5.2)a | 4.7 (4.3-4.9) | 4.6 (4.5-4.8) | |
| SHFJVb | 5.8 (5.5-6.3)c | 5.7 (5.5-6.0)c | 6.2 (5.8-6.8)c | |||
| pH | CO-group | 7.46 (7.43-7.49) | 7.47 (7.45-7.49) | 7.46 (7.43-7.48) | 7.44 (7.4-7.46) | 7.47 (7.44-7.49) |
| PCV | 7.46 (7.44-7.48) | 7.46 (7.44-7.48) | 7.47 (7.45-7.49) | 7.46 (7.44-7.48) | 7.47 (7.45-7.49) | |
| SHFJVb | 7.41 (7.39- 7.43) | 7.4 (7.39-7.41) | 7.38 (7.36-7.39)c |
One animal was excluded from the study because it died of acute cardiac death prior to OLF. None of the animals died during OLF.
The observed values are listed in Table 1. MAP was 10 mmHg higher under SHFJV in the SP compared with PCV [90.0 (IQR: 88.0-91.0) vs 80.0 (IQR: 64.0-89.0) mmHg, P = 0.015]. HR, sPAP, dPAP, and mPAP were slightly increased during SHFJV in the RLP [105.0 (IQR: 99.0-112.0) vs 95.0 (IQR: 91.0-99.0) bpm, P = 0.01; 43.0 (IQR: 38.0-45.0) vs 35.0 (IQR: 33.0-40.0) mmHg, P = 0.03; 25.0 (IQR: 23.0-29.0) vs 21.0 (IQR: 19.0-22.0) mmHg, P = 0.02; 31.0 (IQR: 28.0-34.0) vs 28.0 (IQR: 26.0-29.0) mmHg, P = 0.048], but were normalized after deflooding and TLV.
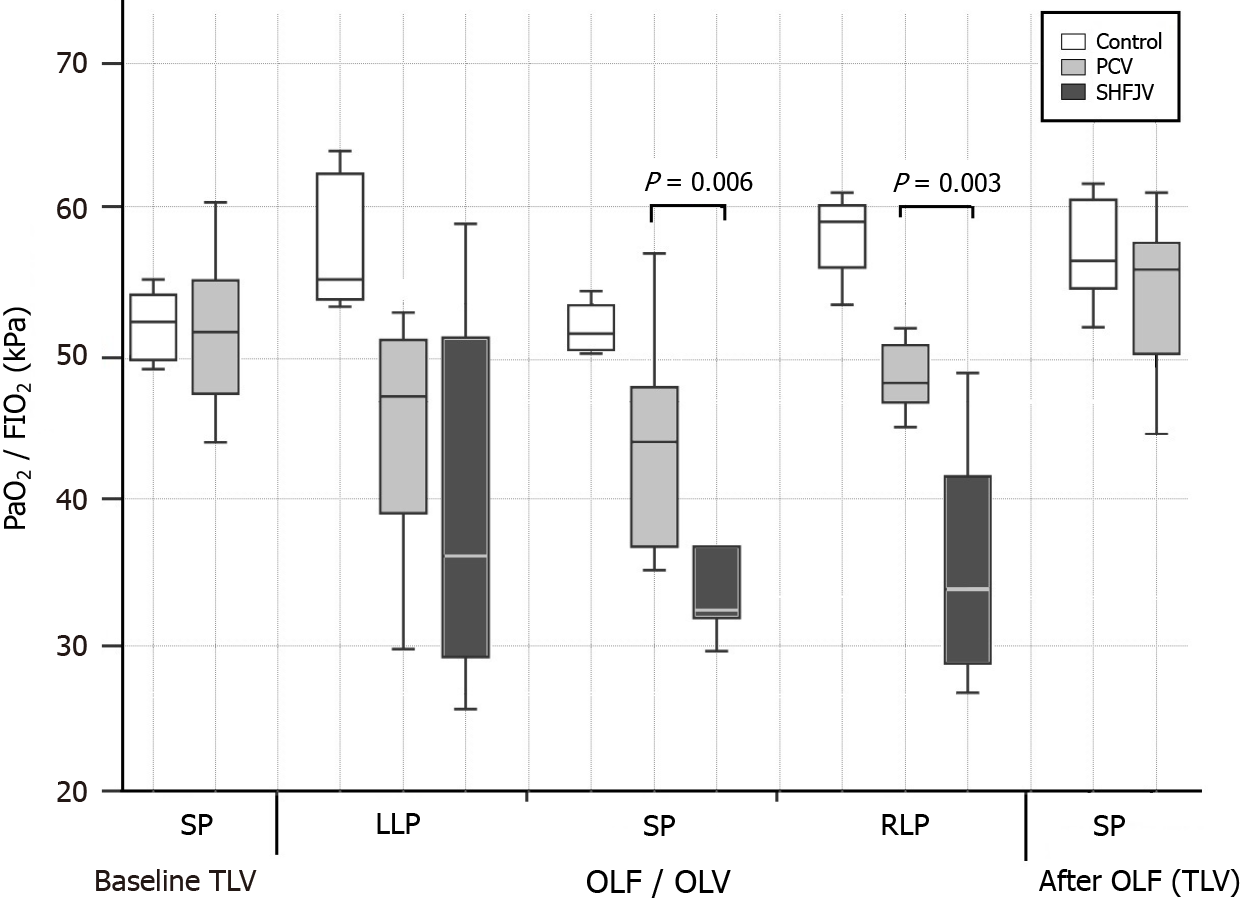
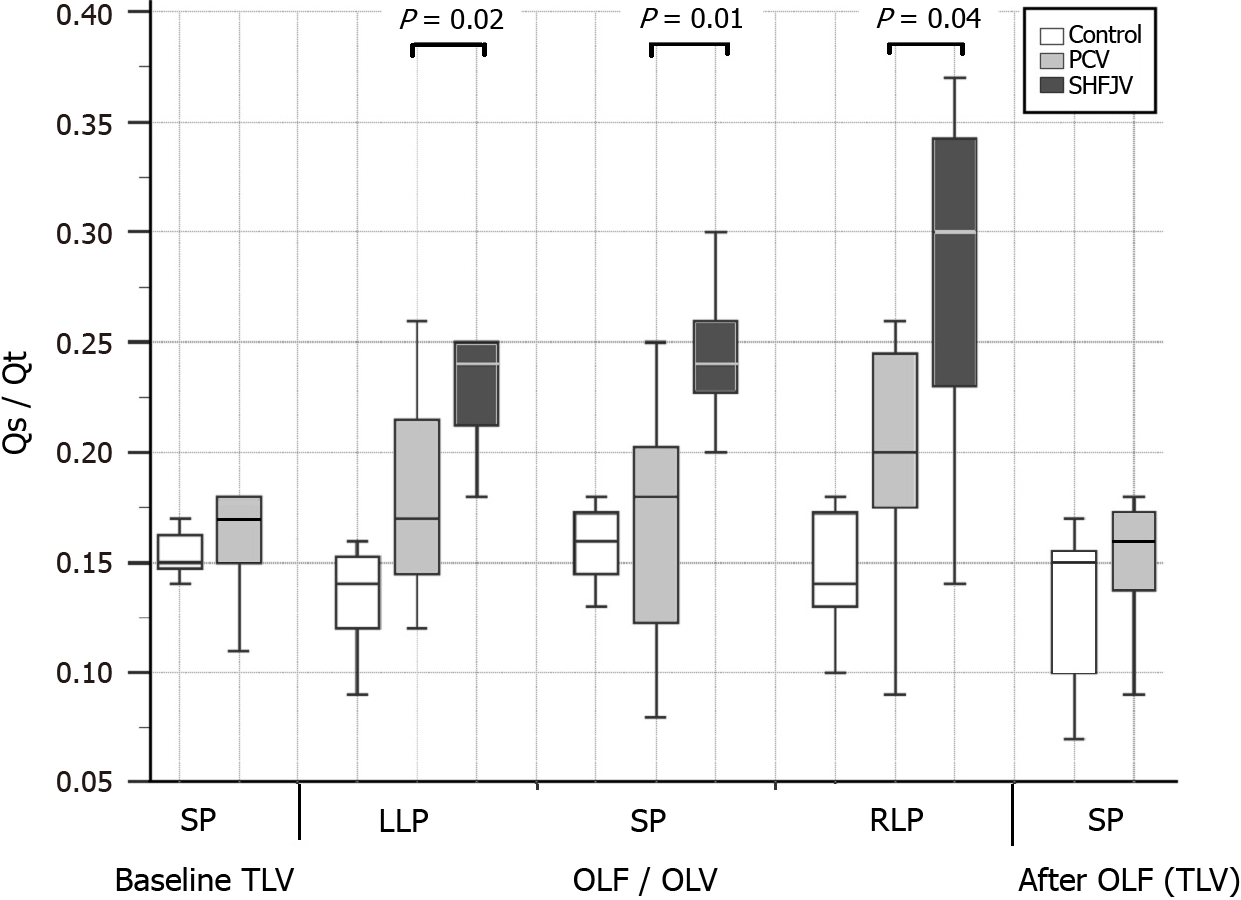
The observed values are presented in Table 2, Figures 5 and 6. Unilateral SHFJV increased PaCO2 compared with PCV in all animal positions with the highest value in the RLP [6.2 (IQR: 5.8-6.8) vs 4.7 (IQR: 4.3-4.9) kPa, P = 0.002]. There was a slightly decreased pH-value in RLP during SHFJV compared with PCV [7.38 (IQR: 7.36-7.39) vs 7.46 (IQR: 7.44-7.48), P = 0.02]. SHFJV slightly decreased oxygenation in the SP and RLP compared to PCV. The lowest PaO2 level was found in the SP [13.0 (IQR: 12.6-15.6) kPa, P = 0.006], the greatest difference between SHFJV and PCV was measured in the RLP [13.6 (IQR: 11.4-17.3) vs 19.8 (IQR: 18.5-20.3) kPa, P = 0.003]. SaO2 was slightly decreased during SHFJV in the SP and RLP compared with PCV [97.6 (IQR: 96.4-98.7) vs 99.7 (IQR: 98.8-99.7), P = 0.01; 97.6 (IQR: 94.3-99.2) vs 99.7 (IQR: 99.7-99.7)%, P = 0.002]. We observed a moderate decrease of PaO2/FiO2 ratio during SHFJV in all animal positions, with the lowest value in SP [32.5 (IQR: 31.5-38.9) kPa, P = 0.006] and the greatest difference between SHFJV and PCV in the RLP [33.9 (IQR: 28.7-43.2) vs 48.0 (IQR: 46.2-50.8) kPa, P = 0.003] (Figure 5). There was an increase in shunt fraction (Qs/Qt) from 0.17 (IQR: 0.14-0.22) to 0.24 (IQR: 0.20-0.25) in LLP (P = 0.02), from 0.18 (IQR: 0.11-0.21) to 0.24 (IQR: 0.22-0.27) in SP (P = 0.01), from 0.20 (IQR: 0.17-0.25) to 0.30 (IQR: 0.22-0.34) in RLP (P = 0.04) under SHFJV compared to PCV (Figure 6). All values were normalized after deflooding and TLV.
Peak inspiratory pressure was 1.7-fold higher under PCV than under SHFJV during OLF [median (min-max): 25 (21.2-40.0) vs 15 (14.0-20.0) cm H2O]. In eight animals, the DP for the low-frequency jet stream during SHFJV increased (between 1.0-1.3 bar) due to the end-expiratory CO2 tension exceeding 45 mmHg. Most often, a pressure increase was necessary for RLP (one animal 1.1 bar, at two animals 1.2 bar, and three animals 1.3 bar).
In all animals, the correct DLT position without dystelectatic lung tissue, particularly in the left upper lobe, was verified (Figure 7A). During OLF, the entire left lung was homogeneous and opaque. The right lung was normally aerated without signs of fluid overflow (Figure 7B). Thirty minutes after OLF, both lungs were aerated normally. Neither dystelectasis nor opacification was observed (Figure 7C).
The present study is the first to investigate the effects of unilateral SHFJV on hemodynamics and gas exchange during OLF in relation to the animal position. We found a slight impairment in gas exchange with the SHFJV compared to the PCV, especially in the RLP (flooded lung above). Considering the well-accepted thresholds for PaO2 (< 8.0 kPa), PaCO2 (> 6.7 kPa), and pH (< 7.35)[29], unilateral SHFJV provides blood gases and hemodynamics in a clinically acceptable range.
Ventilation-induced tumor motion is a significant source of ineffective radiotherapy or percutaneous tumor ablation with HIFU[30,31]. The rationale for using jet ventilation instead of PCV during OLF is to minimize the movement of the flooded lung caused by the contralateral ventilated lung. In a previous study, we showed that SHFJV reduces diaphragm, bronchus, and mediastinal motion compared to PCV during OLF[24]. However, whether unilateral SHFJV ensures adequate gas exchange, especially when a very low DP is needed to maintain low organ movement, is unknown. Based on the pilot study, we chose a DP of 0.9 and 0.4 bar for the low- and high-frequency jet component, which is lower than in the usual clinical use (1.0-3.5 and 0.7-3.5 bar, respectively)[32-34]. The moderate increase of PaCO2 during unilateral SHFJV compared to PCV (6.2 vs 4.7 kPa) can be explained by the low DP of the low-frequency jet component because ventilation (i.e. CO2 removal) is produced by the low frequency. This hypothesis was supported by the measured peak inspiratory pressure under PCV, which was 1.7-fold higher than that under SHFJV. We showed that, in the case of increased end-expiratory CO2 tension, normalization was achieved in a few minutes by a gradual increase in the DP by 0.1 bar from 0.9 to a maximal 1.3 bar. This was necessary six times for eight animals in the RLP. In a pilot study, we found that increasing the DP in the low-frequency mode up to 1.3 bar caused no relevant increase in diaphragm motion.
Unilateral SHFJV resulted in a slight decrease of PaO2 and PaO2/FiO2 ratio in SP (13.0 and 32.5 kPa, respectively) and RLP (13.6 and 33.9 kPa, respectively); however, the lowest values remained in the physiological range. The lowest SaO2 was 97.6%. The lower oxygenation can be explained by the increase in right-to-left shunt perfusion compared to PCV. We found an increase during SHFJV in all animal position (+ 0.07 in LLP, + 0.06 in SP, + 0.10 in RLP, Figure 6). We believe that these findings may be caused by a ventilation/perfusion imbalance in the ventilated lung. On the one hand, pulmonary-arterial perfusion is blocked in the flooded lung, causing a blood diversion from the flooded to the ventilated lung. On the other hand, under SHFJV with low peak inspiratory pressure, dystelectatic lung areas can easily develop, especially because interlobar collateral airways (pores of Kohn and Lambert´s canals) were not found in the lungs of pigs[35]. In the RLP, where the ventilated lung lies below, the chest wall and lung compliance are reduced, and the weight of the flooded lung can accentuate the development of dystelectatic areas in dependent ventilated lungs. The weight of the flooded lung could have less of an impact on humans because pigs do not have a mediastinum comparable to that of humans.
We used an FiO2 of 0.4 in both ventilation methods to avoid absorption atelectasis, as our experimental time was very long. Other studies on SHFJV application in patients and animal models used a higher FiO2 of 0.4[13,14,32]. An increase of FiO2 or DP of the high-frequency jet component is a reliable option to compensate for reduced oxygenation due to dystelectases or atelectases. The unilateral SHFJV resulted in a slight increase in heart rate, systemic arterial pressure, and pulmonary artery pressure, predominantly in the RLP, which can be regarded as clinically irrelevant.
This study has several limitations that should be considered. We compared the two ventilation techniques using a porcine model. Lung volume and lung-chest wall compliance differ from those in adult humans. However, porcine and human cardiorespiratory physiologies are generally quite similar[35]. This study was performed on healthy pigs. The OLF and unilateral SHFJV methods are used for performing focused ultrasound ablation of lung tumors, whereby tumor patients are mostly older and often suffer from chronic obstructive pulmonary disease. Therefore, these results should be interpreted with caution. Our DLT manufactured for pigs was longer and had a smaller ID than the preferred DLT’s for most humans. It should be considered that a higher tube resistance compared with the human DLT could occur[36]. The total duration of the SHFJV was 45 min, but not continuously, because 15 min in each body position was switched to the PCV mode. Thus, the maximum duration of SHFJV that can be safely performed cannot be determined in the present work. We believe that a duration of 15 min is sufficient for HIFU ablation of a lung tumor up to 3 cm in diameter since in a simulation study, 10 min was calculated for the ablation of such a tumor size[37]. The ventilation procedure requires appropriate equipment and experienced users. Finally, to the best of our knowledge, studies on unilateral SHFJV as an OLV method with or without OLF to compare these findings with those of other studies are lacking.
In porcine model, unilateral SHFJV provided adequate ventilation in different animal positions during OLF. The lateral position with the flooded lung above it was the most unfavorable. The very low DPs for the low- and high-frequency jet stream components were due to a compromise between sufficient gas exchange and maximum movement reduction. The slight decrease in oxygenation and CO2 removal compared to PCV did not lead to hypoxia or hypercapnia and was completely reversible. The SHFJV can be safely used for lung tumor ablation to minimize lung motion. The maximum safe duration of SHFJV could not be determined in this study.
Lung cancer prognosis is among the most unfavourable of all cancers. This is reflected in its relatively low 5-year survival rate. Therefore, there is a need to improve local tumour therapy while avoiding surgery. High-intensity focused ultrasound (HIFU) is the only highly effective non-invasive approach to ablating tumours in parenchymal organs outside the chest. A new method one-lung flooding (OLF) for complete lung sonography was developed that allows ultrasound-mediated lung tumour ablation. OLF involves unilateral lung filling with saline, which generates a suitable acoustic pathway for transthoracic application of HIFU in the lung. Saline filling of one lung requires one-lung ventilation of the contralateral lung.
Breathing and lung movement during HIFU procedures can result in incomplete tumour ablation or collateral damage. Superimposed high-frequency jet ventilation (SHFJV) can reduce respiratory motion. However, it is unclear whether unilateral SHFJV allows adequate haemodynamics and gas exchange.
This study aimed to compare SHFJV with pressure-controlled ventilation (PCV) during OLF by assessing hemodynamics and gas exchange relative to the animal position.
SHFJV or PCV were used alternately to ventilate the non-flooded lungs of 12 anaesthetised pigs during OLF in different body positions. Haemodynamic variables and arterial blood gas levels were measured using both ventilation modalities.
Unilateral SHFJV yielded lower carbon dioxide removal than PCV; however, it did not result in elevated carbon dioxide levels. SHFJV exhibited slightly decreased oxygenation in pigs in supine position and RLP compared with PCV. The lowest arterial partial oxygen pressure and arterial partial oxygen pressure/ inspired oxygen fraction (Horowitz index) values were observed in SP [13.0; interquartile range (IQR): 12.6-5.6 and 32.5 (IQR: 31.5-38.9) kPa]. Conversely, SHFJV yielded the highest shunt fractions in all animal positions (highest in the RLP: 0.30).
In this porcine model, unilateral SHFJV may provide adequate ventilation to animals in different positions during OLF. Lower oxygenation and carbon dioxide removal rates compared with PCV did not lead to hypoxia or hypercapnia.
SHFJV can safely minimise ventilation-induced lung motion during lung tumour ablation.
We thank the team at the Central Experimental Animal Facility, Jena University Hospital, especially Mrs. Dobermann, Mrs. Goebel, and Mr. Wuckelt, for their assistance with this project. We thank Dr. Martin Roskos for providing the blood gas analyzer and Dipl. Ing. F. Sick, Medicoplast International GmbH, Illingen, Germany, for providing the double-lumen endobronchial tubes. The authors gratefully acknowledge the technical support provided by Mr. R. Kölbl and Mr. M. Frank (Carl Reiner GmbH, Austria). The authors thank Drs. rer. pol. Thomas Lehmann from the Institute for Medical Statistics, Computer Science, and Data Science, Jena University Hospital, Jena, Germany, for the statistical analyses. The authors gratefully acknowledge the support provided by SRH Wald-Klinikum Gera, particularly PD Drs. U. Leder, MBA, A. Peuke, and M. Lechner.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y, China S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1304] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 2. | Ji Y, Zhu J, Zhu L, Zhu Y, Zhao H. High-Intensity Focused Ultrasound Ablation for Unresectable Primary and Metastatic Liver Cancer: Real-World Research in a Chinese Tertiary Center With 275 Cases. Front Oncol. 2020;10:519164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Fite BZ, Wang J, Kare AJ, Ilovitsh A, Chavez M, Ilovitsh T, Zhang N, Chen W, Robinson E, Zhang H, Kheirolomoom A, Silvestrini MT, Ingham ES, Mahakian LM, Tam SM, Davis RR, Tepper CG, Borowsky AD, Ferrara KW. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci Rep. 2021;11:927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Ning Z, Xie J, Chen Q, Zhang C, Xu L, Song L, Meng Z. HIFU is safe, effective, and feasible in pancreatic cancer patients: a monocentric retrospective study among 523 patients. Onco Targets Ther. 2019;12:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | de Senneville BD, Moonen C, Ries M. MRI-Guided HIFU Methods for the Ablation of Liver and Renal Cancers. Adv Exp Med Biol. 2016;880:43-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Aminzadeh P, Alibrahim E, Dobrotwir A, Paul E, Goergen S. Multiparametric MR evaluation of uterine leiomyosarcoma and STUMP vs leiomyoma in symptomatic women planned for high frequency focussed ultrasound: accuracy of imaging parameters and interobserver agreement for identification of malignancy. Br J Radiol. 2021;94:20200483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Ghai S, Finelli A, Corr K, Chan R, Jokhu S, Li X, McCluskey S, Konukhova A, Hlasny E, van der Kwast TH, Incze PF, Zlotta AR, Hamilton RJ, Haider MA, Kucharczyk W, Perlis N. MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial. Radiology. 2021;298:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | McWilliams JP, Lee EW, Yamamoto S, Loh CT, Kee ST. Image-guided tumor ablation: emerging technologies and future directions. Semin Intervent Radiol. 2010;27:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Brandner ED, Chetty IJ, Giaddui TG, Xiao Y, Huq MS. Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: A review from NRG oncology. Med Phys. 2017;44:2595-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Elen Evans, Peter Biro, Nigel Bedforth. Continuing Education in Anaesthesia Critical Care and Pain. Jet vent. 2007;7:2-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Biro P, Spahn DR, Pfammatter T. High-frequency jet ventilation for minimizing breathing-related liver motion during percutaneous radiofrequency ablation of multiple hepatic tumours. Br J Anaesth. 2009;102:650-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Abderhalden S, Biro P, Hechelhammer L, Pfiffner R, Pfammatter T. CT-guided navigation of percutaneous hepatic and renal radiofrequency ablation under high-frequency jet ventilation: feasibility study. J Vasc Interv Radiol. 2011;22:1275-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Galmén K, Jakobsson JG, Freedman J, Harbut P. High Frequency Jet Ventilation during stereotactic ablation of liver tumours: an observational study on blood gas analysis as a measure of lung function during general anaesthesia. F1000Res. 2019;8:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Galmén K, Jakobsson JG, Perchiazzi G, Freedman J, Harbut P. Quantitative assessment of atelectasis formation under high frequency jet ventilation during liver tumour ablation-A computer tomography study. PLoS One. 2023;18:e0282724. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Biro P, Gottschall R, Klein U, Wiedemann K. Jet-Ventilation. Grundlagen und klinische Anwendung der Jet-Beatmungstechnik. 2001. Available from: https://www.ifm-medical.de/wp-content/uploads/2015/10/booklet-jv-ebook.pdf. |
| 16. | Lesser T, Klinzing S, Schubert H, Klein U, Bartel M. Lung flooding--a new method for complete lung sonography. Res Exp Med (Berl). 1998;198:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wolfram F, Boltze C, Schubert H, Bischoff S, Lesser TG. Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur J Med Res. 2014;19:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Lesser TG, Boltze C, Schubert H, Wolfram F. Flooded Lung Generates a Suitable Acoustic Pathway for Transthoracic Application of High Intensity Focused Ultrasound in Liver. Int J Med Sci. 2016;13:741-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Aloy A, Schachner M, Spiss CK, Cancura W. Tube-free translaryngeal superposed jet ventilation. Anaesthesist. 1990;39:493-498. [PubMed] |
| 20. | Bacher A, Pichler K, Aloy A. Supraglottic combined frequency jet ventilation vs subglottic monofrequent jet ventilation in patients undergoing microlaryngeal surgery. Anesth Analg. 2000;90:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Leiter R, Aliverti A, Priori R, Staun P, Lo Mauro A, Larsson A, Frykholm P. Comparison of superimposed high-frequency jet ventilation with conventional jet ventilation for laryngeal surgery. Br J Anaesth. 2012;108:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kraincuk P, Körmöczi G, Prokop M, Ihra G, Aloy A. Alveolar recruitment of atelectasis under combined high-frequency jet ventilation: a computed tomography study. Intensive Care Med. 2003;29:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Sütterlin R, Priori R, Larsson A, LoMauro A, Frykholm P, Aliverti A. Frequency dependence of lung volume changes during superimposed high-frequency jet ventilation and high-frequency jet ventilation. Br J Anaesth. 2014;112:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Lesser T, Wolfram F, Braun C, Gottschall R. Superimposed High Frequency Jet Ventilation Minimises Diaphragm, Bronchus, and Mediastinum Motion during One-Lung Flooding. Austin J Radiol. 2021;8:1150. [DOI] [Full Text] |
| 25. | Lesser T, Braun C, Wolfram F, Gottschall R. A special double lumen tube for use in pigs is suitable for different lung ventilation conditions. Res Vet Sci. 2020;133:111-116. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Muders T. Einfluss von Spontanatmung auf die regionale Verteilung von Belüftung und Ventilation bei experimentellem Lungenschaden. [cited 4 March 2008]. Available from: https://bonndoc.ulb.uni-bonn.de/xmlui/handle/20.500.11811/3756. |
| 27. | Kneucker A. Funktionelle Residualkapazität und Diffusionskapazität der Lunge bei Kalb und Schwein: Physiologische Werte und Einfluss respiratorischer Infektionen. [cited 14 December 2009]. Available from: https://refubium.fu-berlin.de/handle/fub188/13904. |
| 29. | Miñana G, Núñez J, Bañuls P, Sanchis J, Núñez E, Robles R, Mascarell B, Palau P, Chorro FJ, Llàcer A. Prognostic implications of arterial blood gases in acute decompensated heart failure. Eur J Intern Med. 2011;22:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Bao Y, Zhang L, Fan W, He H, Sun ZW, Hu X, Huang SM, Chen M, Deng XW. Assessment of respiration-induced motion and its impact on treatment outcome for lung cancer. Biomed Res Int. 2013;2013:872739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, Middleton MR. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 401] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 32. | Rezaie-Majd A, Bigenzahn W, Denk DM, Burian M, Kornfehl J, Grasl MCh, Ihra G, Aloy A. Superimposed high-frequency jet ventilation (SHFJV) for endoscopic laryngotracheal surgery in more than 1500 patients. Br J Anaesth. 2006;96:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Hohenforst-Schmidt W, Zarogoulidis P, Huang H, Man YG, Laskou S, Koulouris C, Giannakidis D, Mantalobas S, Florou MC, Amaniti A, Steinheimer M, Sinha A, Freitag L, Turner JF, Browning R, Vogl T, Roman A, Benhassen N, Kesisoglou I, Sapalidis K. A New and Safe Mode of Ventilation for Interventional Pulmonary Medicine: The Ease of Nasal Superimposed High Frequency Jet Ventilation. J Cancer. 2018;9:816-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Veres J, Slavei K, Errhalt P, Seyr M, Ihra G. The Veres adapter: clinical experience with a new device for jet ventilation via a laryngeal mask airway during flexible bronchoscopy. Anesth Analg. 2011;112:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Reinhold P, Rosenbruch M, Theegarten D, Dalhoff K. Pulmonary infection models--7. Workshop on work crises "Comparative pathology and pathophysiology of the respiratory system" of the German Veterinary Medical Society in co-operation with the sections of infectious diseases and tuberculosis and cell biology of the German Society of Pneumology on 17 Mar 2005 om Berlin. Pneumologie. 2005;59:411-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Beermann M, Lindeberg J, Engstrand J, Galmén K, Karlgren S, Stillström D, Nilsson H, Harbut P, Freedman J. 1000 consecutive ablation sessions in the era of computer assisted image guidance - Lessons learned. Eur J Radiol Open. 2019;6:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Wolfram F, Lesser TG. A simulation study of the HIFU ablation process on lung tumours, showing consequences of atypical acoustic properties in flooded lung. Z Med Phys. 2019;29:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |