Copyright
©The Author(s) 2015.
World J Exp Med. Nov 20, 2015; 5(4): 206-217
Published online Nov 20, 2015. doi: 10.5493/wjem.v5.i4.206
Published online Nov 20, 2015. doi: 10.5493/wjem.v5.i4.206
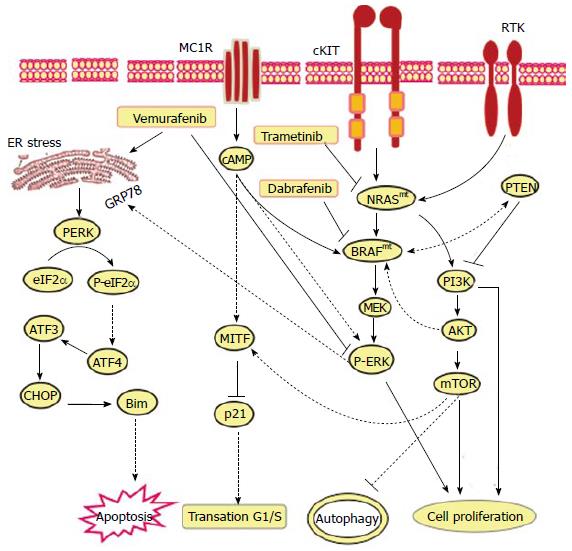
Figure 2 Proposed models for the mechanistic role of endoplasmic reticulum stress in the modulation of the anti-tumor efficiency of vemurafenib, dabrafenib and trametenib in melanoma patients harboring activating neuroblastoma RAS viral (v-ras) oncogene homolog or rapidly accelerated fibrosarcoma murine sarcoma viral (v-raf) oncogene homolog B mutations.
The main function of MC1R, TRK on melanoma cell is to transmit extracellular signaling that is essential for the activation of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways to mediate various cellular functions including melanoma initiation, progression and resistance. Thus, the exposure of melanoma (BRAFmt and NRASmt) cells to either Vemurafenib, Dabrafenib or Trametenib, respectively, results in inhibition of MC1R, cKIT and TRK-mediated activation of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways. As consequence the inhibition of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways results in the execution melanoma cell death via mechanism-mediated by ER stress-dependent pathways including PERK-eIF2α-ATF4-ATF3-CHOP-Bim pathway. NRAS: Neuroblastoma Rat Sarcoma viral (v-ras) oncogene homolog; BRAF: Rapidly Accelerated Fibrosarcoma murine sarcoma viral (v-raf) oncogene homolog B; MC1R: Melanocortin 1 receptor; TRK: CKIT and receptor tyrosine kinase; PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT: Protein kinase B.
- Citation: Hassan M, Selimovic D, Hannig M, Haikel Y, Brodell RT, Megahed M. Endoplasmic reticulum stress-mediated pathways to both apoptosis and autophagy: Significance for melanoma treatment. World J Exp Med 2015; 5(4): 206-217
- URL: https://www.wjgnet.com/2220-315X/full/v5/i4/206.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i4.206