Copyright
©2011 Baishideng Publishing Group Co.
World J Exp Med. Dec 20, 2011; 1(1): 10-16
Published online Dec 20, 2011. doi: 10.5493/wjem.v1.i1.10
Published online Dec 20, 2011. doi: 10.5493/wjem.v1.i1.10
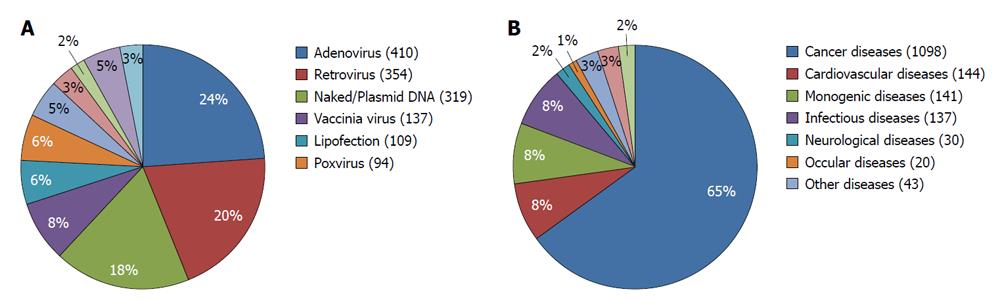
Figure 2 Different gene transfer vectors used in clinical settings (A) and different indications that have been addressed by gene therapy in clinical trials (B).
A: By far, adenoviral, retroviral and naked plasmid/DNA have been the most commonly used gene transfer vectors; B: Even though initial studies have been conducted on monogenetic diseases, cancer soon became a major interest, with 65% of all clinical trials to date. The reasons for this are the highly unmet medical need in cancer therapy as well as its big market size. Also, the ethical acceptance of gene therapy as a therapeutic modality is a factor that has supported the shift from monogenetic diseases to cancer.
- Citation: Wirth T. A short perspective on gene therapy: Clinical experience on gene therapy of gliomablastoma multiforme. World J Exp Med 2011; 1(1): 10-16
- URL: https://www.wjgnet.com/2220-315X/full/v1/i1/10.htm
- DOI: https://dx.doi.org/10.5493/wjem.v1.i1.10