Copyright
©The Author(s) 2021.
World J Crit Care Med. Jan 9, 2021; 10(1): 22-34
Published online Jan 9, 2021. doi: 10.5492/wjccm.v10.i1.22
Published online Jan 9, 2021. doi: 10.5492/wjccm.v10.i1.22
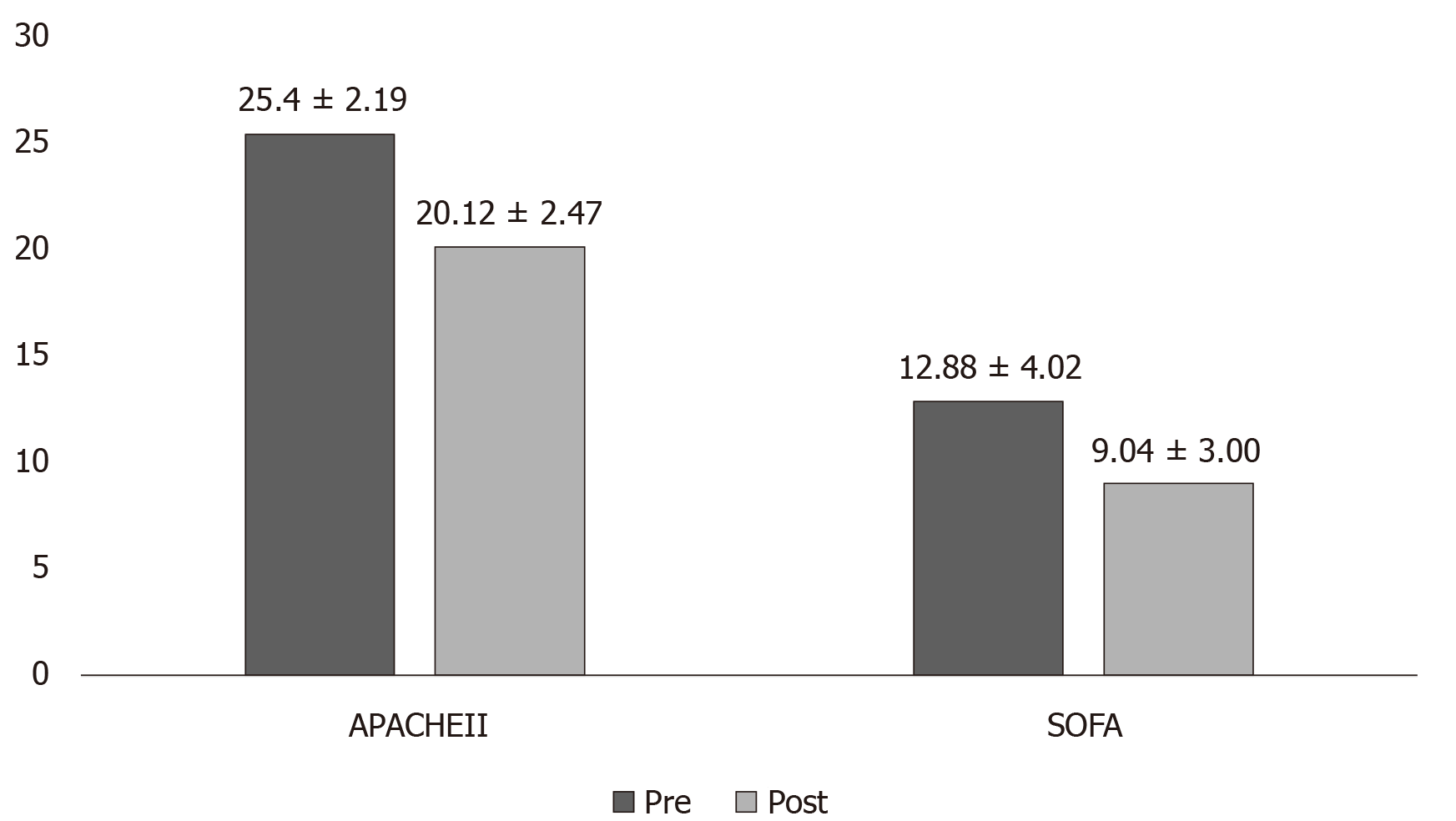
Figure 1 Sepsis scores in survivor group (pre and post Cytosorb® therapy).
Significant P values obtained for both acute physiology and chronic health evaluation (P < 0.0001) and sequential organ failure assessment scores (P = 0.0003). APACHE II: acute physiology and chronic health evaluation; SOFA: Sequential organ failure assessment scores.
- Citation: Paul R, Sathe P, Kumar RS, Prasad S, Aleem M, Sakhalvalkar P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock. World J Crit Care Med 2021; 10(1): 22-34
- URL: https://www.wjgnet.com/2220-3141/full/v10/i1/22.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i1.22