Published online Nov 24, 2022. doi: 10.5412/wjsp.v12.i2.13
Peer-review started: July 25, 2022
First decision: August 22, 2022
Revised: October 12, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: November 24, 2022
Processing time: 122 Days and 2 Hours
Superior mesenteric artery syndrome (SMAS) is a rare condition, characterized by duodenal obstruction caused by compression of its third part by the superior mesenteric artery (SMA). Most cases of SMAS are associated with weight loss, and the most frequent clinical manifestations are nausea, vomiting, postprandial fullness, and abdominal pain. Treatment of SMAS is usually conservative, consist
We describe the case of a man in his forties with a pre-existing diagnosis of esophageal stricture due to sodium hydroxide ingestion, who suffered significant weight loss after replacement of his jejunostomy tube. He was admitted to the hospital due to pain and abdominal distension. A computerized tomography scan showed significant distension of the stomach and duodenum with narrowing of the duodenum at the point at which it is crossed by the superior mesenteric artery, thus establishing the diagnosis of SMAS. Due to the presence of the esophageal stricture, the patient was incapable of emesis; however, passage of a nasogastric tube for decompression was not possible. Considering the risk of gastric perforation due to distention, we opted for surgical treatment in the form of a surgical gastrojejunostomy after which he showed complete resolution of all symptoms and was discharged from the hospital 5 d after the procedure.
Diagnosis of SMAS can be challenging in patients with esophageal stenosis, and risk of gastric perforation may preclude conservative treatment.
Core Tip: We report a rare case of superior mesenteric artery syndrome (SMAS) in a patient with pre-existing esophageal stricture. SMAS is a rare condition that is characterized by duodenal obstruction caused by the compression of its third part by the superior mesenteric artery. While no current evidence-based guidelines to determine ideal treatment for SMAS are available, an initial attempt at conservative treatment with nutritional support aiming at weight gain is indicated in most cases. However, SMAS may present differently in patients with esophageal strictures as the risk of gastric perforation due to ineffective gastric decompression must be considered and may favor early surgical treatment.
- Citation: Barros LCTR, Santos BMRTD, Ferreira GSA, Gomes VMDS, Vieira LPB. Superior mesenteric artery syndrome in a patient with esophageal stenosis: A case report. World J Surg Proced 2022; 12(2): 13-19
- URL: https://www.wjgnet.com/2219-2832/full/v12/i2/13.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v12.i2.13
Superior mesenteric artery syndrome (SMAS), also known as Wilkie’s or cast syndrome, is a rare condition that was first described by Von Rokitansky in the 1860s[1]. The disease is characterized by duodenal obstruction caused by compression of the third part of this organ by the superior mesenteric artery (SMA). The clinical manifestations are those of proximal gastrointestinal obstruction, such as abdominal pain, anorexia, nausea, vomiting, and postprandial fullness. It has an estimated incidence of 0.01% to 0.3% in the general population although given the nonspecific nature of its symptoms, this syndrome is likely underdiagnosed[1].
Regarding its pathophysiology, it is currently accepted that a reduction in the retroperitoneal fat padding leads to the narrowing of the aorto-mesenteric angle and results in duodenal compression by the SMA anteriorly and the aorta and vertebral column posteriorly. The most significant risk factors for SMAS are therefore those related to significant and rapid weight loss (such as severe burns, trauma, bariatric surgery, malignancy)[2]. However, other less frequent causes that can lead to the reduction of the aforementioned angle, such as surgery for correction of spinal scoliosis and restorative proctocolectomy, have also been described[3-6].
As the symptoms are nonspecific, diagnosis may be delayed, which can result in potentially severe complications, such as gastric perforation and peritonitis[7]. Therefore, clinical suspicion of SMAS should be followed by imaging studies to establish the diagnosis. Computed tomography (CT) and magnetic resonance imaging (MRI) are particularly helpful in calculating the aortomesenteric angle[7,8]. A distance between the aorta and the SMA inferior to 10 mm and an aortomesenteric angle of less than 25° are highly suggestive of duodenal obstruction and SMAS[9,10].
We report a rare case of SMAS in a patient with pre-existing esophageal stricture, which presented unique challenges to the diagnosis and treatment of this condition.
A male patient in his forties arrived at the emergency room complaining of abdominal distention and diffuse abdominal pain for the past 4 d.
The patient complained of diffuse abdominal pain that had started 4 d ago and progressively worsened and was associated with malaise and asthenia.
Upon arrival in the emergency room, the patient was hemodynamically stable. His heart rate was 88 beats per minute, blood pressure was 108/76 mmHg, and respiratory rate was 18 breaths per minute. He mentioned having his jejunostomy tube replaced 3 wk before presentation, after which he experienced significant difficulty in diet infusion that resulted in a significant weight loss of 20 kg during that period [weight at admission was 39 kg with a BMI (body mass index) of 13.2 kg/m2].
He was started on intravenous hydration and laboratory examinations were obtained.
The patient had a past medical history of undergoing multiple surgeries for the treatment of corrosive injuries of the upper gastrointestinal tract secondary to ingestion of sodium hydroxide at another hospital 2 years before presenting at our hospital. During that time period, he was suffering from gastric perforation with peritonitis and underwent laparotomy with peritoneal cavity lavage, primary closure of the gastric perforation, and placement of a feeding jejunostomy. He underwent two subsequent procedures for treatment of intra-abdominal abscess and was left with a laparostomy wound that healed secondarily. After this episode, he also suffered from a significant stricture of the distal esophagus, located 20 cm from the incisor teeth, which did not allow a pediatric endoscope or a nasogastric tube to pass through the stricture. While able to drink small amounts of water, he was wholly dependent on enteral feeding through his jejunostomy tube for meeting his nutritional requirements.
The patient had no other comorbidities and reported sporadic use of tobacco, alcohol, and crack cocaine.
Physical examination of the abdomen was remarkable for significant abdominal distention, diffuse pain upon abdominal palpation, and hyper-tympanism on abdominal percussion. An empty rectal vault was noted on digital rectal examination. Vital signs were within the normal range of values, and the patient was afebrile.
Biochemical test results were unremarkable with the only significant finding being a C-reactive protein level of 80 mg/L (reference range: < 10 mg/L). Considering his history of multiple previous abdominal surgeries, intestinal obstruction due to adhesions was considered a likely diagnosis, and the patient underwent CT for further evaluation.
A CT scan demonstrated significant gastric and duodenal distension with formation of air-fluid levels, and marked overinflation of the cuff on the jejunostomy tube.
The jejunostomy tube was then replaced, followed by the outpour of a significant amount of enteric secretion and immediate relief of his symptoms.
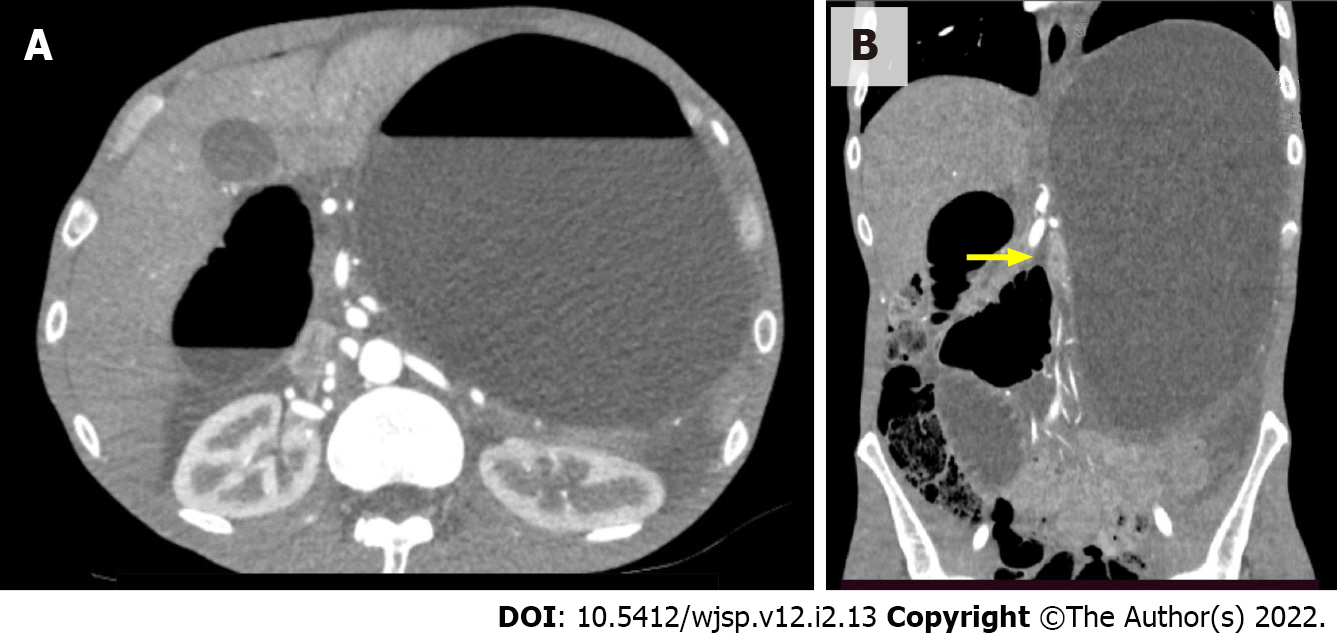
On the following day, however, the patient became symptomatic once again and complained of abdominal pain and distension. Since these symptoms persisted for 2 d, another CT scan was performed for further investigation and revealed gastric and duodenal distension with an abrupt reduction in bowel caliber at the duodenojejunal flexure (Figure 1).
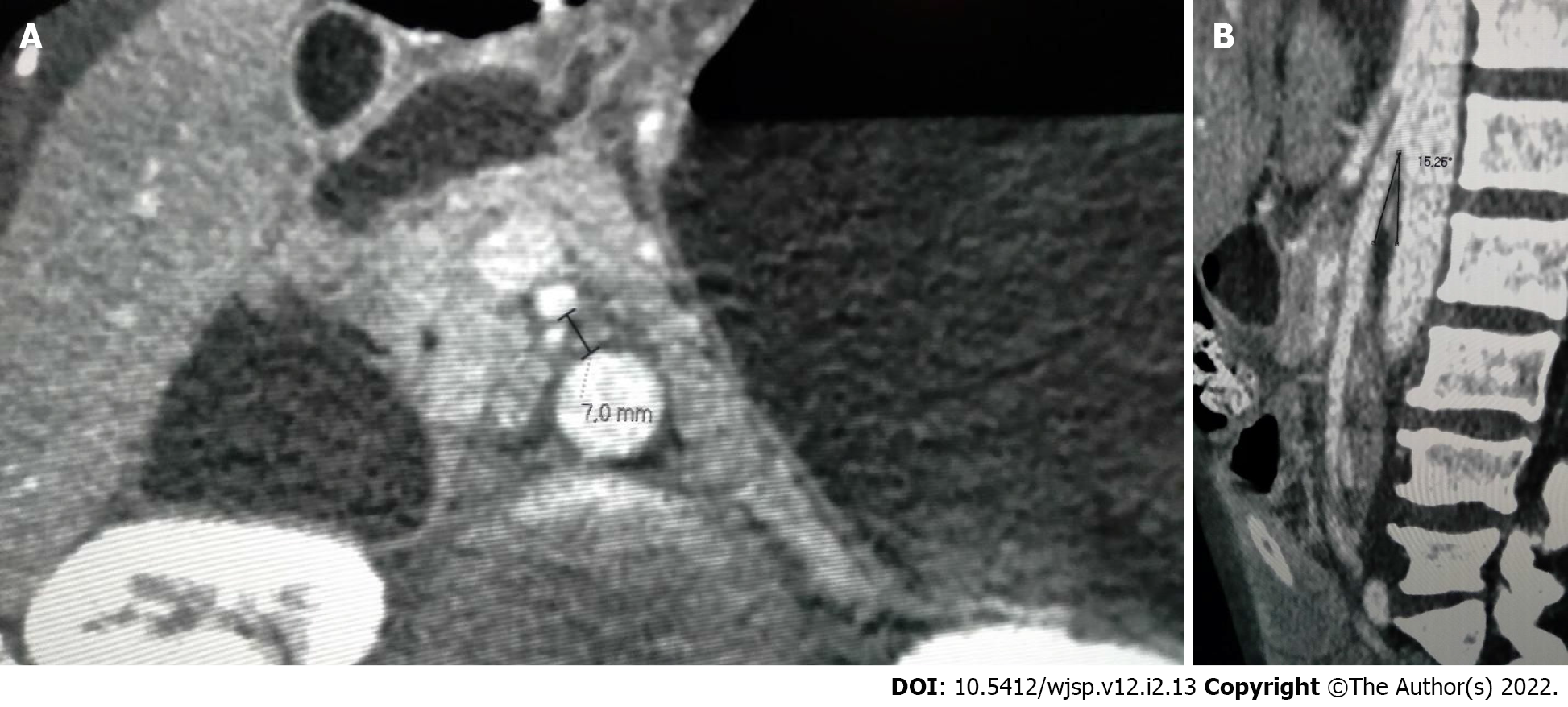
The aortomesenteric angle and distance between the aorta and the SMA were 16.26° and 7 mm, respectively, which corroborated a diagnosis of SMAS (Figure 2). While small bowel obstruction due to adhesions was initially considered as the likely diagnosis, this possibility was excluded by the findings on the second CT scan, which showed dilatation that was restricted to the stomach and part of the duodenum. Overinflation of the cuff on the jejunostomy tube can also be a cause of abdominal pain and distention and was likely the reason for the difficulties faced by the patient during diet infusion. However, as the symptoms recurred after replacement of the jejunostomy tube, another cause for gastric distension was sought. Vomiting is usually one of the hallmark symptoms of SMAS, but patients with esophageal strictures are often incapable of emesis as was the case of the patient in question. Radiographic examinations with the use of oral contrast medium can also be helpful in the diagnosis of SMAS but could not be performed in this case as the patient was intolerant of oral ingestion. The diagnosis of SMAS was obtained after a careful analysis of the CT scan, which showed a marked reduction in the aortomesenteric angle and the distance between the aorta and the SMA. His history of recent and significant weight loss further corroborated the diagnosis of SMAS.
SMAS.
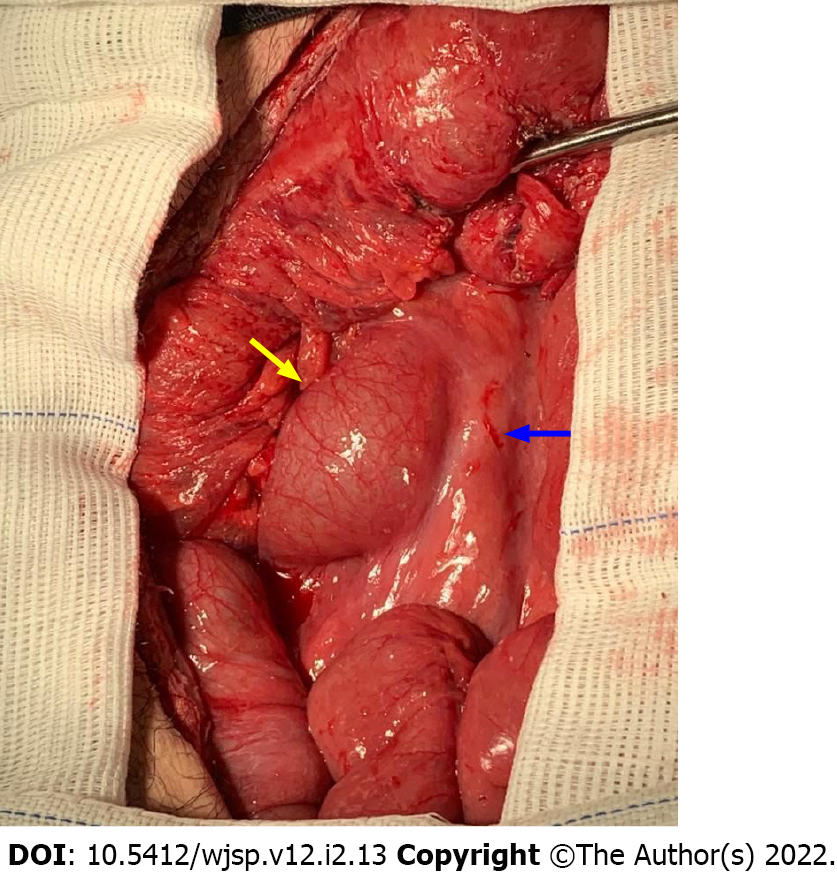
Considering the impossibility of gastric and duodenal decompression with a nasogastric tube due to esophageal stricture and the marked gastric distension viewed on the CT scan, conservative treatment with nutritional support and weight gain was deemed unsuitable for this patient due to risk of gastric perforation with peritonitis. Gastric perforation was considered as a potential complication since there was significant distension of the stomach, which suffered from both proximal (esophageal stricture) and distal (compression by the mesenteric artery) obstruction. We have no access in our institution to guidewire-assisted esophageal dilatation under fluoroscopy, and transfer to an institution with such resources available was not feasible. As the possibilities of surgical treatment were discussed with the patient, he expressed his desire to keep the feeding jejunostomy tube and to undergo no future reconstructive surgical treatments for his esophageal stricture. He then underwent laparotomy, which confirmed the presence of marked gastric and duodenal distention up to the point where the duodenum crossed the aortomesenteric angle with a sudden reduction in bowel caliber (Figure 3). Adhesions from previous surgical procedures were released, and the segment of the jejunum where the jejunostomy tube was placed was resected. A Roux-en-Y gastrojejunostomy was then performed, followed by the placement of a new jejunostomy using the Witzel procedure.
Postoperative recovery was uneventful with hospital discharge after 5 d. The patient remained well and asymptomatic during a follow-up period of 2 mo and has regained 6 kg of weight during that period.
SMAS is a relatively uncommon cause of obstruction of the upper gastrointestinal tract, usually associated with rapid and significant weight loss[1]. Diagnosis is usually based on the assessment of the aortomesenteric angle and the distance between the aorta and the SMA on CT[7,8]. Treatment of SMAS may be conservative or surgical. The conservative approach consists of nutritional support aiming at weight gain and consequently, retroperitoneal fat pad restoration[7]. If not successful, surgery may be needed, especially in cases unrelated to acute weight loss[8,10]. Many surgical approaches, which include gastro- or duodeno-jejunostomy, have been described and can be performed through open surgery or via a laparoscopic approach[11]. Another option for surgical treatment is Strong’s operation, which divides the ligament of Treitz with mobilization of the duodenojejunal junction and repositioning of the duodenum further away from the aorta[7,11]. Duodenal division and re-anastomosis anterior to the SMA is another technique although it is a less commonly used option for the surgical treatment of SMAS[11].
While no current evidence-based guidelines to determine ideal treatment for SMAS are available, an initial attempt at conservative treatment with nutritional support aiming at weight gain is indicated in most cases[7]. Diagnosis and treatment of SMAS require a multidisciplinary approach as a correct radiological diagnosis requires a high index of suspicion, and during the initial stages of treatment, these patients are particularly vulnerable to refeeding syndrome. When conservative treatment fails or is not feasible, surgical treatment with high rates of success is indicated[11]. While scientific evidence is insufficient, laparoscopic gastrojejunostomy or duodenojejunostomy is currently accepted as the standard of treatment as either technique offers optimal decompression of the upper gastrointestinal tract with less surgical trauma[11]. While patients with esophageal obstruction due to neoplasms or benign strictures and who are dependent on gastrostomy or jejunostomy tubes for enteral feeding may be particularly vulnerable to rapid weight loss and therefore to the development of SMAS, we have found no previous report in the literature of SMAS in a patient with pre-existing esophageal stricture. Diagnosis of SMAS in this particular subset of patients may be challenging as characteristic symptoms, such as vomiting and/or postprandial fullness, may be absent. Concern about the lack of an effective mechanism of gastric decompression may lead to gastric perforation and peritonitis, thus causing an increase in the risks of conservative treatment for these patients. While this study is the first report in the literature of SMAS occurring in a patient with esophageal stricture, thus describing a novel situation in the scientific literature, we lack evidence for estimating the risk of potential complications and therefore the ideal treatment for SMAS in the specific settings that we have reported.
SMAS is a rare condition, characterized by duodenal obstruction caused by the compression of its third part by the SMA. While no current evidence-based guidelines to determine ideal treatment for SMAS are available, an initial attempt at conservative treatment with nutritional support aiming at weight gain is indicated in most cases. However, SMAS may present differently in patients with esophageal strictures, as the hallmark symptom of vomiting may be absent. When treating patients with coexisting SMAS and esophageal strictures, the risk of gastric perforation due to ineffective gastric decompression must be considered and may favor early surgical treatment.
The authors would like to acknowledge the important contribution by the radiology team of Hospital Metropolitano Doutor Celio de Castro, in the diagnostic investigation of the case reported, as well as in the elaboration of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Barik R, India; Beji H, Tunisia; Chen C, China; Gica N, Romania; Zhao K, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Yale SH, Tekiner H, Yale ES. Historical terminology and superior mesenteric artery syndrome. Int J Surg Case Rep. 2020;67:282-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ganss A, Rampado S, Savarino E, Bardini R. Superior Mesenteric Artery Syndrome: a Prospective Study in a Single Institution. J Gastrointest Surg. 2019;23:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Sapkas G, O'Brien JP. Vascular compression of the duodenum (cast syndrome) associated with the treatment of spinal deformities. A report of six cases. Arch Orthop Trauma Surg (1978). 1981;98:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zadegan F, Lenoir T, Drain O, Dauzac C, Leroux R, Morel E, Guigui P. [Superior mesenteric artery syndrome following correction of spinal deformity: case report and review of the literature]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11:3307-3310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 6. | Matheus Cde O, Waisberg J, Zewer MH, Godoy AC. Syndrome of duodenal compression by the superior mesenteric artery following restorative proctocolectomy: a case report and review of literature. Sao Paulo Med J. 2005;123:151-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Zaraket V, Deeb L. Wilkie's Syndrome or Superior Mesenteric Artery Syndrome: Fact or Fantasy? Case Rep Gastroenterol. 2015;9:194-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Welsch T, Büchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007;24:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Neto PRF, Paiva RA, Filho AL. Superior mesenteric artery compression syndrome - case report. J Coloprocto. 2011;31:401-404. [DOI] [Full Text] |
| 10. | Ghimire S, Yousef MA, Gondal A. Superior mesenteric artery syndrome: case report and review of literature. Amer J Gastroenterol. 2020;115:pS1448. [DOI] [Full Text] |
| 11. | Kirby GC, Faulconer ER, Robinson SJ, Perry A, Downing R. Superior mesenteric artery syndrome: a single centre experience of laparoscopic duodenojejunostomy as the operation of choice. Ann R Coll Surg Engl. 2017;99:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |