Copyright
©The Author(s) 2015.
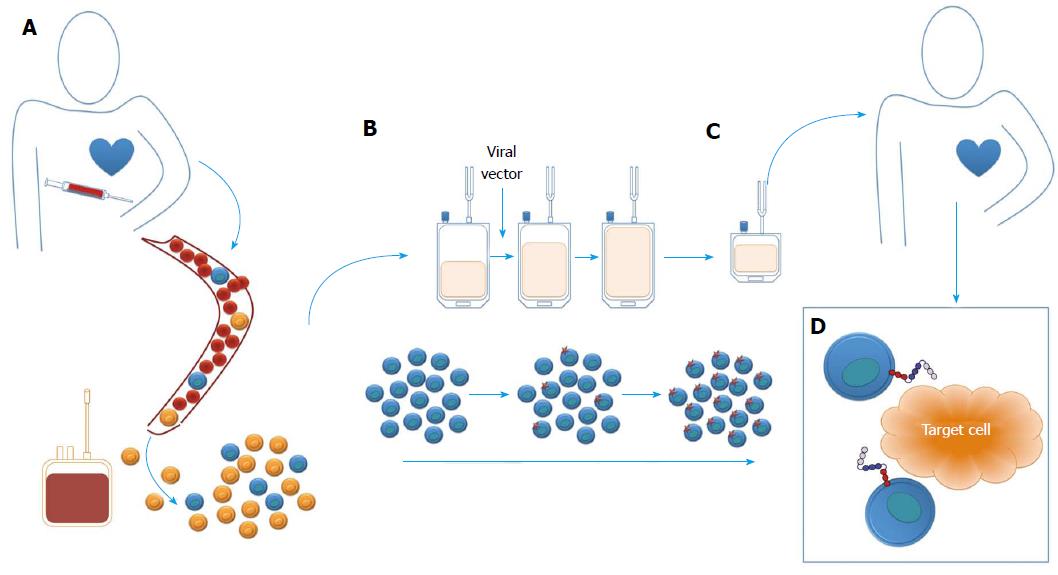
Figure 2 The overview of manufacture of chimeric antigen receptor T-cell products.
A: Starting material is most commonly a leukapheresis, although some systems employ whole blood at this stage; B: Peripheral blood mononuclear cells are isolated and T-cells then activated and genetically engineered (in this case with a viral vector); C: After ex-vivo expansion, the cell product is formulated and administered; D: After which engineered cells can engage target cells via the CAR. CAR: Chimeric antigen receptor.
- Citation: Schalkwyk MCV, Maher J. Chimeric antigen receptors: On the road to realising their full potential. World J Immunol 2015; 5(3): 86-94
- URL: https://www.wjgnet.com/2219-2824/full/v5/i3/86.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i3.86