Copyright
©2013 Baishideng Publishing Group Co.
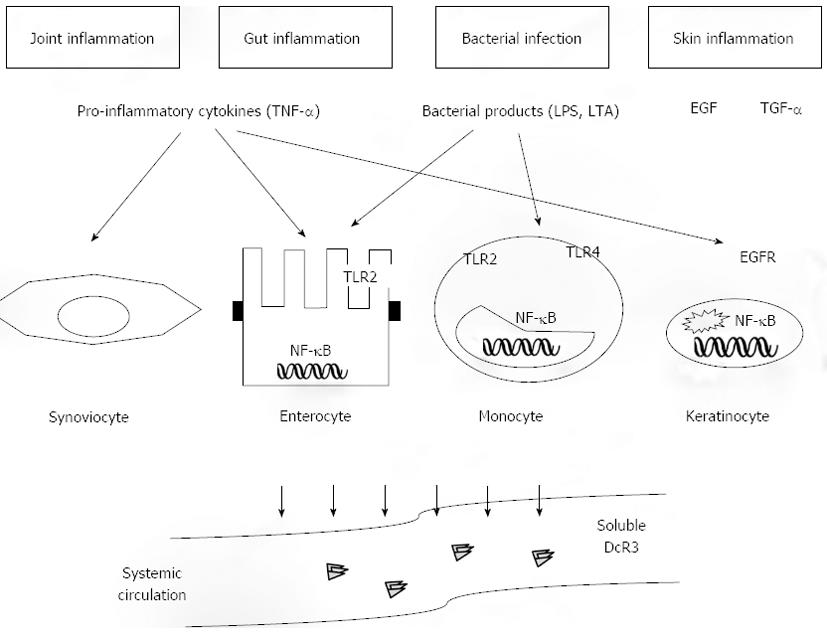
Figure 2 Dual immunomodulatory function of Decoy receptor 3.
Decoy receptor 3 (DcR3) displays several properties that are of pathogenetic relevance during inflammatory reactions. The final effect may be either protective or pathogenic depending on several parameters, such as the specific condition (autoimmunity vs infection) and the cell type (lymphocyte, monocyte, epithelial cell). The majority of these effects are associated to the function of DcR3 as a decoy receptor, which inhibits signalling via the tumor necrosis factor superfamily ligands FasL, lymphotoxin-like inducible protein that competes with glycoprotein D for herpesvirus entry on T cells (LIGHT), and tumor necrosis factor-like molecule 1A (TL1A), through competition for ligand binding with the respective functional receptors Fas, herpesvirus entry mediator (HVEM), and DR3. DcR3 inhibits apoptosis induced by ligand/receptor interactions, which may have either deleterious or beneficial effects depending upon the specific cellular component. In particular, blockade of apoptosis in activated effector lymphocytes would lead to perpetuation of chronic inflammatory injury. On the other hand, it may induce the survival of intestinal epithelial cells in inflammatory bowel disease, leading to tissue protection. DcR3 may also interfere with effector immunological responses in several ways. It blocks LIGHT/HVEM- or TL1A/DR3-mediated co-stimulation of activated lymphocytes, leading to impaired proliferation and defective cytokine production. Such an effect may have direct anti-inflammatory function during autoimmune diseases. On the opposite, in the event of an infection such defects in effector lymphocyte activation may lead to impaired clearance of the microorganism and enhanced severity of the infection. The same duality takes place in relation to the effect of DcR3 on dendritic cell differentiation towards a Th2-polarizing antigen-presenting-cell subtype, which is a non-decoy function, as it occurs independently of binding to FasL, LIGHT or TL1A. Such Th2 polarization may dampen Th1-type pro-inflammatory responses in autoimmunity, but may lead to defective anti-microbial function during infections. An inverse correlation may occur for the effects of DcR3 on monocytes that include upregulation of the adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion protein-1 and enhanced adherence to endothelial cells. This may offer an advantage during infection via the recruitment of inflammatory cells; at the same time, however, it may be deleterious during autoimmune inflammation as it may lead to the constant homing of monocytes to the inflammatory foci and perpetuation of tissue injury. ICAM-1: Intercellular adhesion molecule-1; VCAM-1: Vascular cell adhesion protein-1.
- Citation: Siakavellas SI, Bamias G. Decoy receptor 3: Its role as biomarker for chronic inflammatory diseases. World J Immunol 2013; 3(3): 44-53
- URL: https://www.wjgnet.com/2219-2824/full/v3/i3/44.htm
- DOI: https://dx.doi.org/10.5411/wji.v3.i3.44