Published online Aug 24, 2022. doi: 10.5411/wji.v12.i2.15
Peer-review started: May 2, 2022
First decision: May 31, 2022
Revised: June 9, 2022
Accepted: August 17, 2022
Article in press: August 17, 2022
Published online: August 24, 2022
Processing time: 112 Days and 1.7 Hours
Free radicals (reactive oxygen species, superoxides and hydroxyl radicals) lead to the development of oxidative stress because of imbalance in the amount of antioxidants. Continued development of oxidative stress leads to chronic diseases in humans. The instability in the antioxidant activities and accumulation of oxidative stress due to free radicals may occur in diseases like inflammatory bowel disease (IBD). Antioxidants are substances that inhibit or delay the mechanism of oxidation of molecules mediated by free radicals and also transform into lesser-active derivatives. Probiotics are defined as live microorganisms that show beneficial effects on inflamed intestine and balance the inflammatory immune responses in the gut. Probiotic strains have been reported to scavenge hydroxyl radicals and superoxide anions that are abundantly produced during oxidative stress. The most widely studied probiotic strains are Streptococcus, Bifidobacterium and Lactobacillus. Probiotics cultured in broth have shown some amount of antioxidant activities. Fermented milk and soy milk, which possess starter microorganisms (probiotics), tends to increase the antioxidant activities many-fold. This review aims to discuss the in vivo and in vitro antioxidant activities of specific probiotics with various assays with respect to IBD.
Core tip: Inflammatory bowel diseases (IBDs) are degenerative diseases that cause chronic inflammation in the intestine. The most prevalent therapy for IBD is conventional antibiotic therapy. Keeping the adverse effects of antibiotics in mind, researchers have shown that Streptococcus, Lactobacillus and Bifidobacterium are some of the most efficient antioxidative agents with respect to in vitro and in vivo activities. Probiotics individually or in combination play an important role in regulating superoxide dismutase activity, which is always dysregulated due to oxidative stress caused in IBD. The mechanism of antioxidation of probiotics using NRf2-antioxidative response element pathway, nuclear factor-B and protein kinase C pathway may be activated to contribute to the reduction of oxidative-stress-induced IBD. The review focuses on the antioxidative activities of the specific bacterial strains as therapeutic molecules in IBD. Multiple combinations of probiotic strains have still not been adequately studied. We are currently researching the antioxidative effect of Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacterium bifidumin combination.
- Citation: Biswas S, Ray Banerjee E. Probiotic treatment of inflammatory bowel disease: Its extent and intensity. World J Immunol 2022; 12(2): 15-24
- URL: https://www.wjgnet.com/2219-2824/full/v12/i2/15.htm
- DOI: https://dx.doi.org/10.5411/wji.v12.i2.15
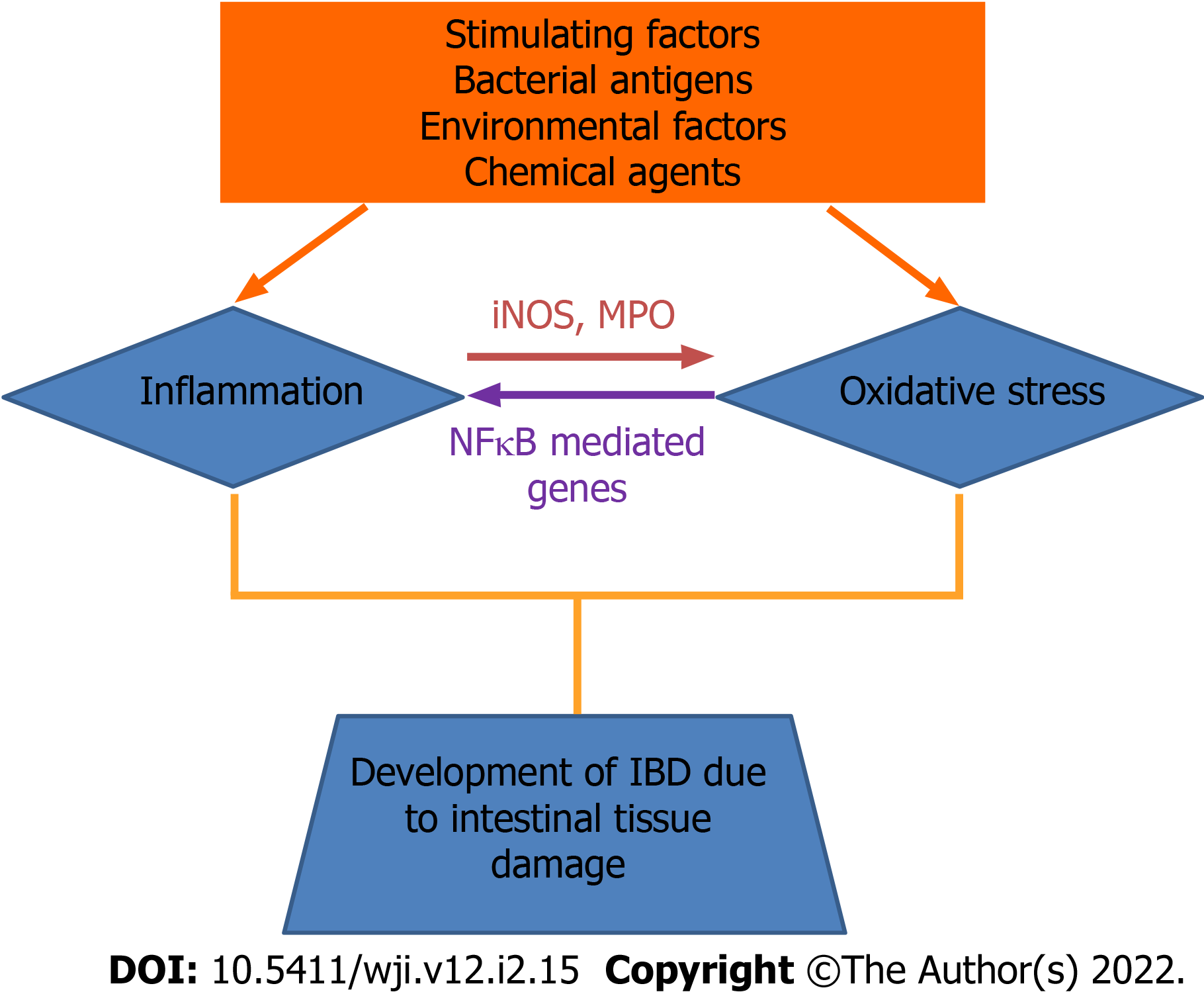
Inflammatory bowel disease (IBD) is an umbrella term used to describe chronic inflammation in the human digestive tract. IBDs are characterized by diarrhea, rectal bleeding, abdominal pain, fatigue and weight loss. IBDs are prevalent in western countries, although they are on the rising track in the Asian countries, which mimics the prevalence in American and European countries. When the burden of IBD is compared between eastern and western countries, the prevalence of IBD in India, which is one of the eastern countries, is found to be the highest. The imbalance in pro-oxidants and antioxidants in the gut leads to inflammation. Despite having antibiotic medication, the prevalence of IBD is still high worldwide. Thus, there is a need to investigate small molecule therapeutic approaches to stop the increase in the number of cases of IBD. In humans, reactive oxygen species (ROS) function as regulators and mediators to ensure correct cell functioning[1,2]. Overproduction of ROS can easily induce damage to proteins, nucleic acids or lipids through free radical reactions. Therefore, in the event of excess ROS production, protective antioxidant mechanisms are required to prevent oxidative stress[1,2]. ROS include superoxides, nitric oxides (NO), hydroxyl radicals, singlet oxygen and hydrogen peroxide (H2O2) that contributes to cellular damage, leading to inflammation. IBD is known for the occurrence of oxidative stress. Ulcerative colitis (UC), which is one type of IBD, leads to the increased generation of highly toxic ROS that exceeds the capacity of the limited intestinal antioxidative defense system[3,4]. Oxidative stress in IBD is the key factor for progression of inflammation and is identified by the increased production of ROS, decreased antioxidant molecules and enzymes (beta-carotene, vitamin C and vitamin E) and enhanced lipid peroxidation in the intestine[5].
In the inflammatory processes, intestinal cells of inflamed tissue in response to chemical agents or pathogens, produce high levels of ROS and superoxide anions[6]. Exposure to antigens for a short period of time does not cause any harm because of the adequate first-line defense system producing antioxidative enzymes for protection[6]. However, in chronic intestinal inflammation, there is persistent high ROS production. This process damages the intestinal epithelial barrier, enhances inflammation and injures the intestinal epithelium[6]. Lipid peroxidation is another process that involves a source of secondary free radicals, which directly interact with other biomolecules. The lipid peroxidation depends on the number of double bonds; therefore, polyunsaturated fatty acids are the most susceptible to oxidation. Lipid peroxidation occurs on polyunsaturated fatty acids located on the cell membrane[7]. Superoxide anion radicals, H2O2 and hydroxyl radicals secreted by neutrophils and other phagocytes, causes cell membrane to be impaired, eventually leading to cell death by lipid peroxidation[6]. Enhanced free radicals in the gut can exert peroxidation of membrane phospholipids of intestinal epithelial cells, resulting in the release of toxic products like malondialdehyde (MDA) that can cause damage and cellular stress. MDA is the key breakdown product of lipid peroxides, which is present in the plasma of IBD patients[6]. Increased level of MDA in plasma of Crohn’s disease (CD) patients is considered to be anoxidative stress marker[6]. Decreased superoxide dismutase (SOD)-2 expression is one of the identification markers in colitis-induced mice.
The current preferred therapies for IBD include 5-aminosalicylate, steroids, corticosteroids and azathioprine[8]. The limitations of IBD therapy include the clinical adverse effects of antibiotics, corticosteroids and immunomodulators, which revolves around nausea, vomiting, stomach pain, diarrhea, headaches, respiratory infections, acne, weight gain, insomnia, dizziness, muscle or joint cramps and pathological side effects, causing some pathogenic bacteria to become resistant in IBD. Surgery is generally costly and unaffordable to many people in remote areas. Also it can cause harm to many organs. Thus, the literature reviews have confirmed the apparent need for improvised treatment using small molecules, like probiotics[9]. Nowadays, 60%–80% of the world population relies on alternative medication to cure IBD. Probiotics are preferably of human origin: they have to be safe for the host, genetically stable and capable of surviving throughout the gastrointestinal tract. Probiotics are generally applicable for viable cells, whereas, postbiotics are soluble factors (either secreted by live bacteria or released after bacterial cell lysis), which are beneficial to human hosts. Probiotics have recently been emerged as one of the powerful novel therapeutic small molecules against IBD. They have been shown to have a positive effect on oxidative stress by promoting the potency of the antioxidative defense system, and in turn may lower the risk of several inflammatory disorders such as IBD. Various known probiotics play an important role in antioxidative activity. Probiotics could be a possible intervention for reducing ROS and lipid peroxidation and thereby increasing SOD activity. Our goal was to review on the in vivo and in vitro antioxidative activities of probiotics. Antioxidative activities of probiotics like Streptococcus, Bifidobacterium and Lactobacilli against oxidative stress in IBD are the main focus of the review.
Oxidative stress occurs due to an imbalance between free radical production and antioxidant defense, resulting in hydroxylation of DNA, denaturation of proteins, peroxidation of lipid, and apoptosis, ultimately compromising cell viability[10]. An excess of oxidative stress can lead to the oxidation of lipids and proteins, which is associated with changes in their structure and function. H2O2 is formed by dismutation of superoxides or direct reduction of oxygen. H2O2 can penetrate most of the cell membranes and react with iron in the cell to form hydroxyl radicals. Therefore, hydrogen peroxides are more cytotoxic than superoxide anion radicals. The oxidative modification of lipids, proteins, nucleic acids and carbohydrates is induced and mediated by both free radicals and nonradical activities of reactive species[7,11]. Superoxides are unreactive molecules but undergo dismutation or enzymatic catalysis to form H2O2[7,11]. Hydroxyl radicals are thought to initiate ROS and remove hydrogen atoms. This form of radical is extremely reactive and attack most cellular components[7,11] (Figure 1).
To neutralize the damaging effect of oxidative stress, we need supplements that possess some antioxidative activities. Antioxidants are proteins or enzymes in nature. Antioxidants inhibit cellular damage mainly through their radical scavenging properties[12]. The principle micronutrients that can scavenge free radicals are vitamin E, Vitamin C and beta-carotene. Humans cannot produce these antioxidant micronutrients. So, they must be supplied through the diet[7]. SOD catalyzes the breakdown of superoxide anions into oxygen and H2O2 using Zn/Cu, Fe/Mn and Ni as cofactors[10,13]. Only a few species of Lactobacillus, Lactobacilluscasei, Lactobacillusparaplantarum, Lactobacillusbucneri and Lactobacillussakei exhibit SOD activity. Catalases are the common enzymes found in all living organisms, which are frequently used by cells to catalyze the decomposition of H2O2to water and less reactive gaseous oxygen[10].
The nicotinamide adenine dinucleotide phosphate (NADP) oxidase/NADP peroxidase enzyme system prevents oxygen accumulation in bacterial cells by formation of H2O2 followed by water. This maintains an intracellular redox balance[10,14]. Antioxidants work by scavenging free radicals, preventing production of free radicals and improving levels of endogenous antioxidants. Scavenging antioxidants remove active species rapidly, before they react with biologically essential molecules in the body. This antioxidants function by scavenging active free radicals before they attack biologically essential molecules by donating hydrogen atoms to give stable compounds.
When the antioxidant capacity of damaged mucosa is compromised, various natural substances can act as antioxidant molecules to inhibit ROS generation, cell damages and improve the activity of antioxidative enzymes in cells. A food can be considered as functional, when it is demonstrated to provide nutritional effects for health and well-being and reduction of the risk of disease. Ingredients that make foods functional are: dietary fibers, vitamins, minerals, antioxidants and essential fatty acids. One of the novel approaches as therapy against oxidative stress are the development of probiotics[16,17]. Probiotics are the functional foods that possess antioxidant properties[7,15]. Several studies have highlighted that the ability of probiotics are to enhance antioxidant properties. For probiotics growth, milk can be used as a substrate for starter microorganisms. Naturally, milk has its own antioxidant activities due to the presence of bioactive compounds of whey proteins, caseins, lactoferrin, urate, ascorbate, alpha-tocopherol, beta-carotene as well as enzymes like SOD, catalase and glutathione peroxidase. Fermented milk with probiotic microorganisms has further improved antioxidant potential[18]. Furthermore, the fermentation of soyabean extract using probiotic cultures of lactic acid bacteria possesses superoxide radical scavenging and reducing activities. Soybeans contain SOD, which possesses the superoxide anion scavenging effect. Soymilk obtained from soybean is also expected to possess SOD. The fermented soymilk has an increased superoxide-anion-scavenging effect due to the production of secretory byproducts in the presence of lactic acid bacteria[19].
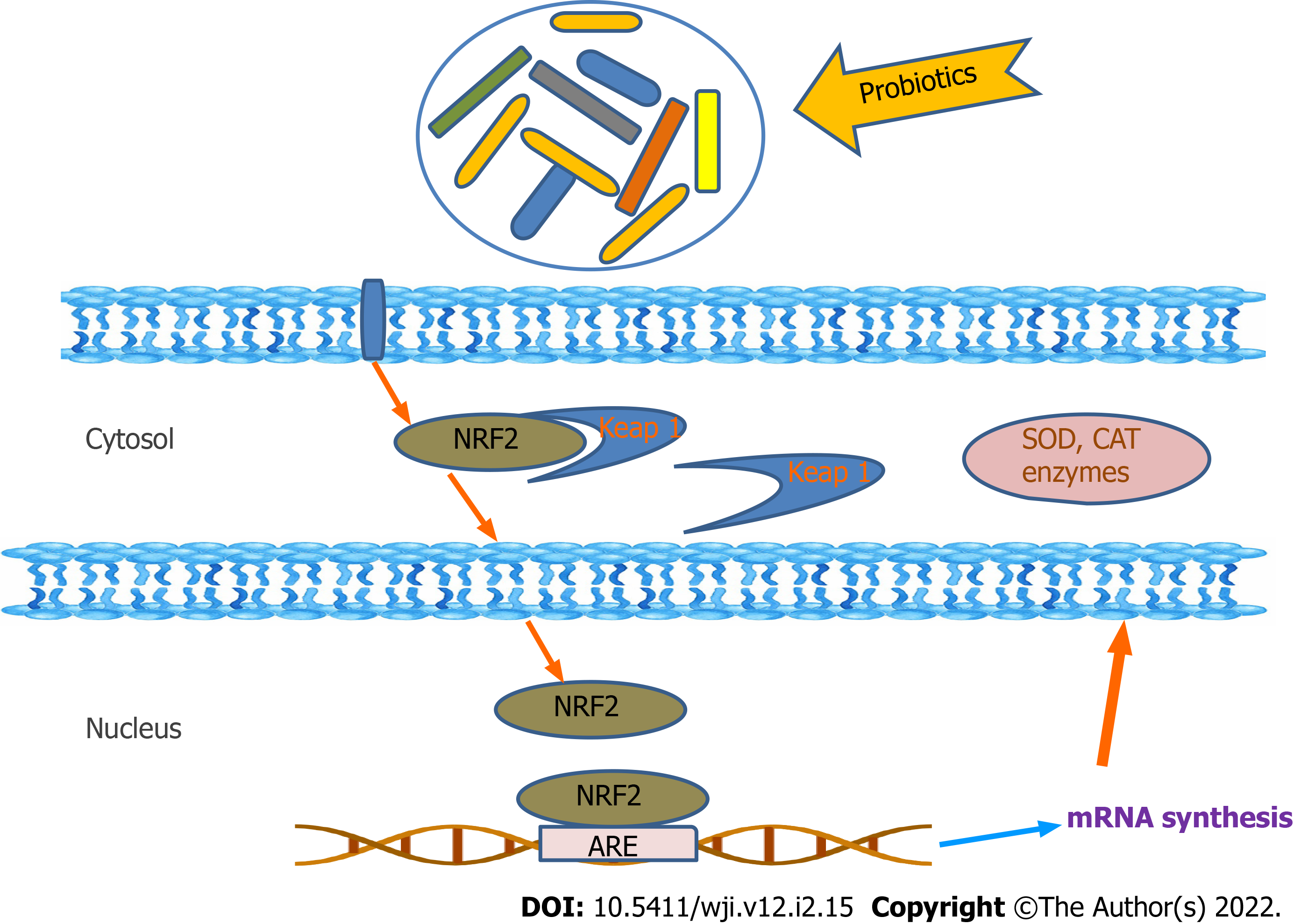
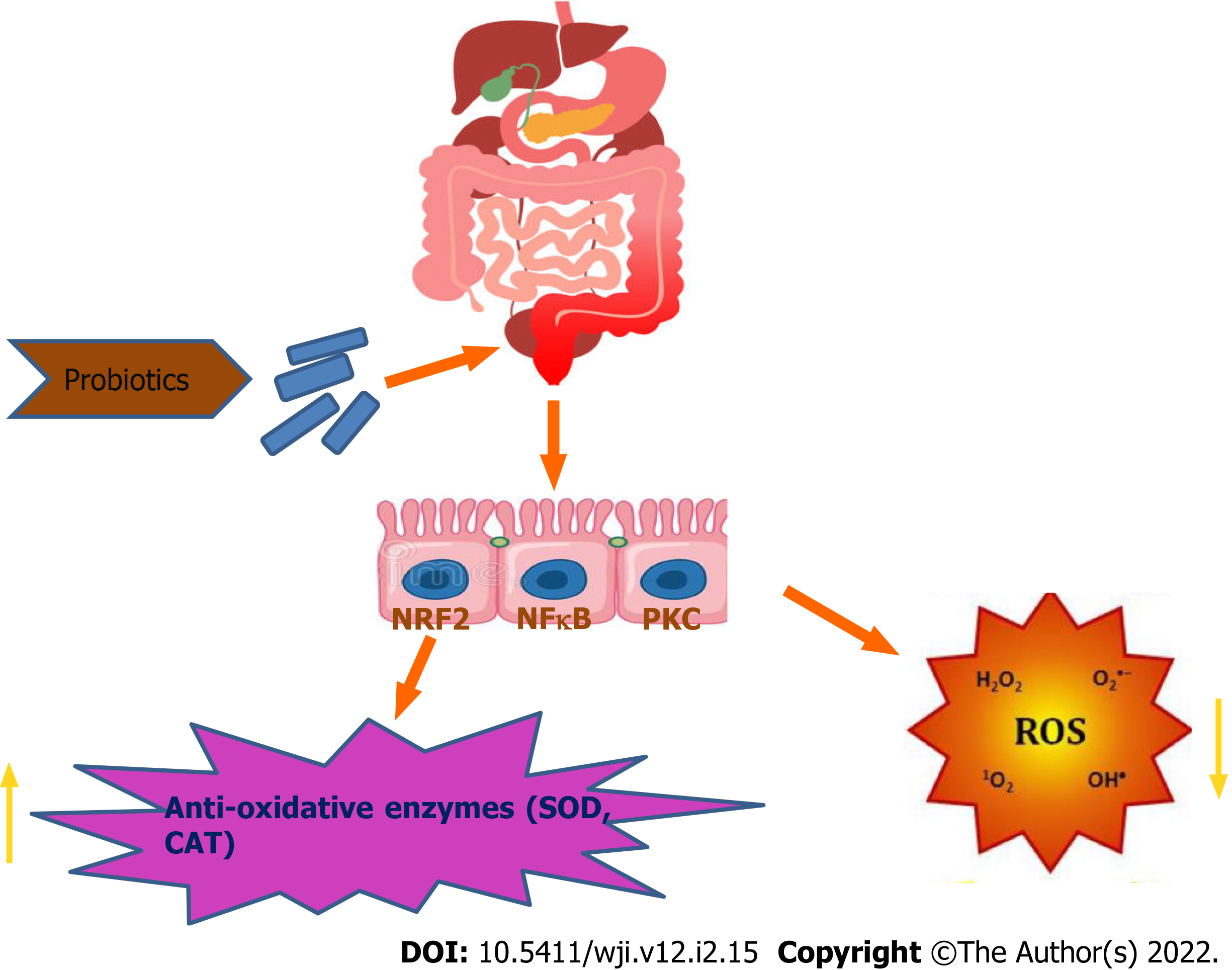
Probiotics can directly act to neutralize oxidants by the production of antioxidant enzymes. The antioxidant mechanism of probiotics could be assigned to ROS scavenging, chelation of metal ions, enzyme inhibition and their reducing ability. Probiotics have an antioxidant effect by scavenging of oxidants or by prevention of generation of free radicals in the intestine. Probiotics can upregulate the intracellular activity of SOD, catalase and glutathione peroxidase to protect the cells from intracellular damage. Pro-oxidative metal ions are capable of initiating decomposition of H2O2 into radicals and triggering lipid peroxidation. Certain chelators are normally detected in probiotics, stating the chelating capacity of probiotics[8,18]. According to reviews, Lactobacillus rhamnosus and Lactobacillus paracasei have significantly inhibited the production of hydrogen peroxide, whereas, L. casei also possess high antioxidant activity via chelating Fe2+[10,21]. Different in vitro and in vivo studies have reported that probiotic bacteria can protect against oxidative stress through regulation of the Nrf2 (Nuclear factor erythroid 2-related factor 2)–Keap1–antioxidant response element (ARE) pathway, protein kinase C (PKC) pathway and nuclear factor (NF)-B pathway[7,10,22].
The Nrf2–Keap1–ARE system transmits signal into the nucleus. Under normal conditions, Keap1 is associated with Nrf2. However, in ROS infiltration in cells, the bond between Keap1 and Nrf2 is cleaved and Nrf2 eventually enters the nucleus and binds to ARE and enhances the production of the antioxidative enzymesproduction[7,10,23]. ROS activates NF-B, entailing expression of inflammatory cytokines. NF-B responds to oxidative stress. Thus, the probiotic formulations (Lactobacillussp., Bifidobacteriumsp. and Streptococcussp.) are able to inhibit NF-B activation in colonicepithelial cells[10,24] (Figures 2 and 3). PKCs are the family of protein kinases that are the target for redox modifications. Administration of L. plantarumimproved the oxidative stress in a rat model of obstructive jaundice by strengthening the expression and activity of the PKCpathway[10,24,25].
Not all the probiotics have antioxidant activity due to high strain heterogeneity. Bacillus proteolyticus shows the highest 1-diphenyl-2-picrylhydrazyl (DPPH) and hydroxyl radical scavenging activity[26]. Zeng et al[26] reported that Bacillus amyloliquefaciens could significantly increase the antioxidative capacity of epithelial cells to reduce induced oxidative stress in pigs. Bacillus subtilis and L.casei can scavenge free radicals (in vitro) and reduce oxidative damage by improving lipid metabolism followed by reduction in lipid peroxidation. Streptococcus thermophilus (YIT 2001) showed the highest in vitroantioxidative activity against lipid peroxidation[27]. Lactobacillus and S.thermophilus showed the highest TAALA (total antioxidant activity against linoleic acid oxidation) and TAAAA (total antioxidant activity against ascorbate auto-oxidation). The cell-free extracts and intact cells of Lactobacillus acidophilus (ATCC4356) demonstrated an increased inhibition of linoleic acid peroxidation from 38% to 48%. This indicates astrongantioxidativeactivity[14]. Bifidobacterium longum was also investigated for inhibition of lipid peroxidation activity.
Stress induced HT29 cells, i.e., H2O2-stimulated HT29 cells showed a reduced amount of intracellular SOD, catalase and increased ROS activity. The cultured cells were treated with probiotics for 24 h. The supernatant of the cells was collected to study the presence of the antioxidative enzyme activity of SOD and catalase[28]. The Bifidobacterium bifidum treated cell line showed increased catalase activity. SOD and catalase production by B. bifidum can decrease oxidative stress. Moreover, in vitro studies have showed that strains like L. acidophilus and Lactobacillus delbrueckii displayed highest superoxide anion radical dismutation. L. plantarum showed increased ability to degrade chemically pure H2O2 and demonstrated the highest catalase activity[29]. SOD activity was found in Lactococcus, S. thermophilus and Bifidobacterium, with significantly higher activity in Lactococcus than in S. thermophilus[14]. SOD activity of cell-free extracts of the above-mentioned probiotics was studied by the amount of inhibition of reduction of nitrobluetetrazolium[14]. Greatest SOD activity was demonstrated by Lactococcus strains. Glutathione was analyzed in deproteinized bacterial cell-free extract using a commercial kit that showed that the Lactococcus group had the highest inhibitory effect[14]. However, S.thermophilus, Lactococcus lactis and Bifidobacterium animalis also contained relevant amounts of intracellular reduced and oxidized forms of glutathione. Total glutathione measurement was carried out in presence of glutathione reductase and NADP[14].
In an animal model of IBD, it was observed that L. acidophilus with dismutase-like activity was more effective than L. plantarum in suppressing the inflammatory process[29]. In vivo studies have also revealed that L.plantarum 0B and L. acidophilus has the highest catalase activity and highest dismutase-like activity respectively. Male Wister rats were administered with probiotic formulation (mixture of B.animalis, L. acidophilus DSMZ 23033 and Lactobacillus brevis DSMZ 23034) after acclimatization of rats in cages. After 18 d of probiotics supplementation, blood plasma was collected to study the antioxidant status[14]. Reactive oxygen metabolite (ROM) concentration of plasma was evaluated as studied by d-ROM test. Plasma total antioxidant activity (TAA) was spectrometric ally measured in the presence of 2-binamine-di-3-ethylbenzothiazolin-6-sulfonic acid (ABTS) radical by evaluating the decoloration and reduction of radical cations of ABTS[14]. Plasma ROM concentration was inversely related to the dose of administered probiotics[14]. TAA was significantly related to the dose of administered probiotics. In another study, oral administration of Bifidobacterium breve yakult appeared to prevent transepidermal water loss and significantly suppress oxidation of lipids, proteins and H2O2 levels[31].
The antioxidant activity of buffalo milk fermented with B.bifidum and L.acidophilus was evaluated. Control groups included mice fed with standard dahi without probiotic enrichment and another with fermented milk. Catalase and SOD activity in blood was analyzed[27,31]. SOD activity in red blood cells increased exclusively after probiotic dahi administration. Dahi supplemented by L.casei NCDC19 and L.acidophilus NCDC14 inhibited lipid peroxidation and maintained the activity of glutathione peroxidase, SOD and catalaseinstreptozotocin-induced oxidative stress inrats[32,33]. Lactobacillus fermentum (Lf1) was studied to assess its antioxidative properties, and confirmed the enhanced expression of NRF2 and MDA inhibition in HT29 cells under stress[34]. In another study it was shown that S.thermophilus YIT2001 decreased the amount of lipid peroxide in colonic mucosa and improved the symptoms of DSS-induced colitis in mice[27].
Scavenging activity of ROS is one of the antioxidative properties of probiotics. The Reactive Oxygen Species are used to include both oxygen centered radicals and nonradical derivatives of oxygen. There is the scavenging activity of probiotics occurs in conditions where there is abundance of ROS, hydroxyl radicals and H2O2.
To evaluate the antioxidative activity of probiotics, DPPH solution was mixed with methanol and probiotic sample and incubated at 37 degree Celsius for 30 min in the dark. The DPPH radical scavenging activitywascalculated by measuring the absorbance of the sample and blank at 517 nm.The radical scavenging activity was calculated as follows: [1-(A517 (sample)/A517 (blank)]× 100%. According to Das and Goyal, DPPH radical scavenging activity was higher in L. plantarum and L. acidophilus. Scavenging activity of Bacillus ranged from 46% to 190%. B. proteolyticus showed the highest DPPH radical scavenging activity, whereas, B. amyloliquefaciens had the weakest DPPH radical scavenging activity[36]. Probiotic strains such as S. thermophilus and L. delbrueckii can scavenge ROS, hydroxyl radicals and H2O2[37]. Cell-free supernatants of Lactobacillus exhibit strong DPPH radical scavenging activity[37]. Moreover, the crude peptides extracted from L. acidophilus, L. casei and L. paracaseihave radical scavenging activities for DPPH in vitro.
To study the effectiveness of antioxidants, inhibition of lipid peroxidation is commonly studied. Bacterial strains (L. acidophilus and B. longum) and the intracellular cell-free extract indicated an inhibitory rate on linoleic acid peroxidation that ranged from 33% to 46%[38]. L. acidophilus and B. longum demonstrated a high antioxidative activity for inhibiting lipid peroxidation. Inhibitory rate of different strains of L. acidophilus ranged from 34.9% to 46.3%[37]. Cell-free supernatants of Lactobacillus show higher inhibitory effect than MRS broth cell culture. Intact cells or intracellular cell-free extracts of L. acidophilus and B. longum were investigated for their antioxidative effects, which demonstrated that inhibition of linoleic acid peroxidation ranged from 38% to 48%[34,39]. Levilactobacillus brevis exhibited greater radical scavenging activity and lipid peroxidation inhibitory activity than Pediococcus pentasaceus[35]. Many studies related to lipid peroxidation have chosen linoleic acid as the source of unsaturated fatty acids. Unsaturated fatty acids such as linoleic acid, methyl linoleate and arachidonic acid are typically used. The protocol forlipid peroxidation assay using linoleic acid has been standardized to study the inhibition of linoleic acid peroxidation. Egg homogenate is generally not used for lipid peroxidation inhibition studies in the presence of probiotics. Thus, lipid peroxidation assay using egg homogenate can be used to investigate the inhibition of lipid peroxidation by probiotics.
Reducing power is based on the kinetics of reduction of Fe3+ to Fe2+ to prevent the oxidation reaction[37]. Ferric-reducing antioxidant power allows estimation of the ability to reduce pro-oxidant metal ions. The fermented black soybean broths of B. subtilis have shown a potent reducing power as compared to positive controls i.e., -tocopherol and Butylated hydroxytoluene[39]. Cell-free supernatants of Lactobacillus strains showed significantly higher reducing power than MRS broth containing Lactobacillus[38]. Ferric ion reducing antioxidant power assay was performed for the fermented milk with Lactobacillussp., S.thermophilus and Bifidobacteriumsp. in the presence of green tea supplementation[15]. Fermented milk with 15% green tea infusion (GTI) shows the highest anti-oxidative power as compared to 10% or 5% GTI[15].
Superoxides are radicals with free electrons located on oxygen[16]. These radicals initiate lipid oxidation as the superoxides and H2O2 are precursors of singlet oxygen and hydroxyl radicals[17]. Assays can measure the ability to scavenge superoxide anion radicals. S. thermophilus containing fermented milk accounts for the highest superoxide anion scavenging effect as compared to L. acidophilus. Archibald and Fridovich showed that S. thermophilus was able to produce SOD, while L.acidophilus was not. Fermented soy milk with L. acidophilus+Bifidobacterium infantis, L. acidophilus+B. longum, S. thermophilus+B. infantis, or S. thermophilus+B. longum shows higher superoxide anion scavenging activity than reducing activity[17]. The cell-free supernatant of L.plantarum and L.acidophilus showed a potent inhibitory superoxide radical scavenging activity with increasing concentration compared to ascorbic acid[40]. Xing et al[36] had studied an enhanced superoxide radical scavenging activity in co-fermentation conditions in milk (with B.infantis, L. plantarum, B. animalis and S. thermophilus). S. thermophilus exhibited only 58.34% activity, whereas co-fermentation increased the superoxide scavenging activity to 65%.
H2O2 can be generated in biological system in oxidative stress conditions. Being a non-radical oxygen containing reactive agent, it can form hydroxyl radicals (the most highly oxygen radical known). Soymilk fermented with Bifidobacterium alone accumulated the largest amount of H2O2, whereas, fermented soymilk with Bifidobacterium and lactic acid bacteria simultaneously reduced H2O2[17].
Among reactive oxygen species, hydroxyl radicals are the most reactive species. It can react with polyunsaturated fatty acid moieties of cell membrane phospholipids and causes damage to the cells. Venkatesan et al stated that different concentrations of probiotic species of Bifidobacterium and Lactobacillus showed strongest radical scavenging activities. The hydroxyl radical scavenging activity of cell-free supernatant of L.plantarum and L. acidophilus showed potent hydroxyl radical scavenging activity when compared to positive control ascorbic acid. These two specific strains have shown a better DPPH and hydroxyl radical scavenging activity. The radical scavenging activity was calculated as follows: [A(sample)-A(control)/A(blank)-A(Control)]× 100%.Cell-free supernatants of various Lactobacillus strains (L. rhamnosus, L. casei, L. plantarum, L. reuteri, L. acidophilus, Lactobacillus fermenti and Lactobacillus parciminis) were studied through invitro cell-free hydroxyl radical assay. It was concluded that all the Lactobacillus strains showed a better scavenging than hydroxyl radical scavenging activities.
Generally, antioxidants are molecules that interact with the free radicals generated in the cells and terminate the chain reaction before damage is done to the vital molecules. In recent years, researchers have witnessed a beneficial effect of probiotics, especially in regulating the oxidative stress in IBD[32]. Lactobacillus, Streptococcus and Bifidobacterium have been shown to have antioxidative activity that can easily scavenge oxidative stress inducing molecules inside a cell.
From this review, it can be concluded that, in IBD, high levels of oxidative stress induce intestinal tissue damage. Oxidative stress is defined as an imbalance between pro-oxidants and antioxidants, and is tightly associated with the exacerbation of IBD. This disturbs the cellular homeostasis by causing cell injury and increased permeability of the mucosal barrier. Probiotics are equipped with antioxidative defense mechanisms, not only to protect their own survival but also to confer protection to the host cell against oxidative stress during colitis. Probiotics are used to combat IBD by reducing ROS generation and lipid peroxidation and by increasing production of antioxidant enzymes (SOD, catalases and peroxidases)[40]. The most common strains studied, Bifidobacterium and Lactobacillus are reported to secrete SOD and antioxidant molecules that can alleviate oxidative stress in inflamed intestine[41]. Accumulation of probiotic strains in inflamed colon results in some protective effects like, metal-chelating activities, antioxidant enzymes (SOD), eventually showing free-radical scavenging activities by restoring the gut microbiota during colitis. Different in vitro studies have suggested that combination of probiotics in fermented milk improve its antioxidative activity[40]. An enhanced superoxide radical scavenging activity of soy milk containing Bifidobacterium was observed. Multiple in vivo and in vitro studies have demonstrated that Lactobacillus, Streptococcus and Bifidobacterium possess outstanding antioxidant activities like DPPH, hydroxyl, superoxide radical scavenging and reducing activities. The important mechanism of antioxidant activities used by probiotics is to reduce oxidative stress, which includes, redox signaling of Nrf2 leading to increase in antioxidant enzyme levels and scavenging of Reactive Oxygen Species. Moreover, it can also be concluded that multiple probiotic strains in combination is much more effective than single probiotic strain with respect to antioxidative studies. Antioxidant probiotic strains can be selected and investigated as promising candidate against IBD. Thus, to develop a novel probiotic combination product with the potential for preventing the oxidative stress, there remains a need to search for particular probiotic strains that can be effective in mitigation of oxidative stress in IBD. The molecular mechanism of the reviewed probiotic strains (Streptococcus, Lactobacillus and Bifidobacterium) by which they regulate the oxidative stress based cellular cascade in IBD conditions needs to be investigated in detail and validate these antioxidative properties in specific in vivo models. Likewise, our novel combination probiotics (S. thermophilus, L. acidophilus and B. bifidum) are under investigation with respect to their antioxidative properties.
I would like to thank my guide professor Ena Ray Banerjee for mentoring the whole review work. I am also thankful to the Department of Zoology, University of Calcutta, and all my esteemed professors, who inspire us to work ahead and succeed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J, China; Xu G, China S-Editor: Xing YX L-Editor: Kerr C P-Editor: Xing YX
| 1. | Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3054] [Article Influence: 234.9] [Reference Citation Analysis (1)] |
| 2. | Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res ClinObstetGynaecol. 2011;25:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 706] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 3. | Kim DH, Cheon JH. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017;17:25-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Krzystek-Korpacka M, Kempiński R, Bromke MA, Neubauer K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Alzoghaibi MA, Al Mofleh IA, Al-Jebreen AM. Lipid peroxides in patients with inflammatory bowel disease. Saudi J Gastroenterol. 2007;13:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3066] [Cited by in RCA: 2556] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 7. | Averina OV, Poluektova EU, Marsova MV, Danilenko VN. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | JainM, Venkataraman, Jayanthi Inflammatory bowel diseaseAn Indian Perspective. Indian Journal of Medical Research 2021; 153:421-430. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Park SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. 2022;20:159-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J ClinBiochem. 2015;30:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1402] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 11. | Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood). 2012;237:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 12. | Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free RadicBiol Med. 2010;49:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 13. | Serata M, Yasuda E, Sako T. Effect of superoxide dismutase and manganese on superoxide tolerance in Lactobacillus casei strain Shirota and analysis of multiple manganese transporters. Biosci Microbiota Food Health. 2018;37:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Naraki S, Igimi S, Sasaki Y. NADH peroxidase plays a crucial role in consuming H2O2 in Lactobacillus casei IGM394. Biosci Microbiota Food Health. 2020;39:45-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol. 2013;97:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 325] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 16. | Najgebauer-Lejko D. Effect of green tea supplementation on the microbiological, antioxidant, and sensory properties of probiotic milks. Dairy Sci Technol. 2014;94:327-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Rossi M, Amaretti A. Probiotic properties ofBifidobacteria: genomics and molecular aspects. Horizon Scientific Press, UK, 97–123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Hoffmann A, Kleniewska P, Pawliczak R. Antioxidative activity of probiotics. Arch Med Sci. 2021;17:792-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Wang YC, Yu RC, Chou CC. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Lee J, Hwang KT. Chung MY, Cho DH, Park CS. Resistance of Lactobacillus casei KCTC 3260 to Reactive Oxygen Species (ROS): Role for a Metal Ion Chelating Effect. J Food Sci. 2005;70: 388-391. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Tao F, Jing W. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes. 2020;12:1801944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 22. | Wang LX, Liu K, Gao DW, Hao JK. Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol. 2013;19:3150-3156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 23. | Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free RadicBiol Med. 2000;28:1349-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 529] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 25. | Zhou YK, Qin HL, Zhang M, Shen TY. Effects of Lactobacillus plantarum on gut barrier functions in experimental obstructive jaundice. World J Gastroenterol. 2012;14:3977-3991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Zeng Z, He X, Li F, Zhang Y, Huang Z, Wang Y, Li K, Bao Y, Iqbal M, Fakhar-E-AlamKulyar M, Li J. Probiotic Properties of Bacillus proteolyticus Isolated From Tibetan Yaks, China. Front Microbiol. 2021;12:649207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ito M, Ohishi K, Yoshida Y, Okumura T, Sato T, Yokoi W, Sawada H. Preventive effect of Streptococcus thermophilus YIT 2001 on dextran sulfate sodium-induced colitis in mice. BiosciBiotechnolBiochem. 2008;72:2543-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Lin Z, Ku S, Lim T, Park SY, Park MS, Ji GE, O'Brien K, Hwang KT. Antioxidant and Anti-Inflammatory Properties of Recombinant Bifidobacterium bifidum BGN4 Expressing Antioxidant Enzymes. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Lin MY, Yen CL. Reactive oxygen species and lipid peroxidation product-scavenging ability of yogurt organisms. J Dairy Sci. 1999;82:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Tomusiak-Plebanek A, Heczko P, Skowron B, Baranowska A, Okoń K, Thor PJ, Strus M. Lactobacilli with superoxide dismutase-like or catalase activity are more effective in alleviating inflammation in an inflammatory bowel disease mouse model. Drug Des DevelTher. 2018;12:3221-3233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Ishii Y, Sugimoto S, Izawa N, Sone T, Chiba K, Miyazaki K. Oral administration of Bifidobacterium breve attenuates UV-induced barrier perturbation and oxidative stress in hairless mice skin. Arch Dermatol Res. 2014;306:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Li W. Antioxidant Properties of Probiotic Bacteria. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 33. | Kaushal D, Kansal VK. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum alleviates age-inflicted oxidative stress and improves expression of biomarkers of ageing in mice. Mol Biol Rep. 2012;39:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Chauhan R, Vasanthakumari AS, Panwar H, Mallapa RH, Duary RK, Batish VK, Grover S. Amelioration of colitis in mouse model by exploring antioxidative potentials of an indigenous probiotic strain of Lactobacillus fermentum Lf1. Biomed Res Int. 2014;2014:206732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Yang SJ, Kim KT, Kim TY, Paik HD. Probiotic Properties and Antioxidant Activities of Pediococcuspentosaceus SC28 and Levilactobacillus brevis KU15151 in Fermented Black Gamju. Foods. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Xing J, Wang G, Zhang Q, Liu X, Gu Z, Zhang H, Chen YQ, Chen W. Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: a comparison with traditional methods. PLoS One. 2015;10:e0119058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Lin MY, Yen CL. Inhibition of lipid peroxidation by Lactobacillus acidophilus and Bifidobacterium longum. J Agric Food Chem. 1999;47:3661-3664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. 2000;45:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 257] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Lin CC, Wu PS, Liang DW, Kwan CC, Chen YS. Quality, antioxidative ability, and cell proliferation-enhancing activity of fermented black soybean broths with various supplemental culture medium. J Food Sci. 2012;77:C95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Li SN, Tang SH, He Q, Hu JX, Zheng J. In vitro antioxidant and angiotensin-converting enzyme inhibitory activity of fermented milk with different culture combinations. J Dairy Sci. 2020;103:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |