INTRODUCTION
Exchange transfusion (ET) is a definitive and effective therapy for preventing kernicterus, usually where intensive phototherapy is either lacking or proves to be ineffective in arresting rapidly rising bilirubin levels in infants with severe neonatal hyperbilirubinemia or symptoms of acute bilirubin encephalopathy (ABE)[1,2]. The procedure is not risk-free however, as it may be associated with such complications as sepsis, electrolyte imbalance, air embolism, portal vein thrombosis, cardiac overload, thrombophlebitis, thrombocytopenia, necrotizing enterocolitis, and the transmission of blood-borne diseases, even in settings with advanced clinical care[3-6]. Several guidelines for the management of neonatal hyperbilirubinemia in developed and developing countries recommend immediate ET for infants with, or at risk of, acute or chronic bilirubin encephalopathy[2,7,8]. This is primarily because the timing of ET vis-à-vis the complex interaction between the level and duration of exposure of the neuronal cells to unbound bilirubin crucially affects intervention outcomes[9]. However, this timely goal is rarely achieved in many low- and middle-income countries (LMICs), where excessive rates of ET persist as a result of weaknesses in the health-care delivery system in these locations[10-13]. For example, it is not uncommon for a severely jaundiced infant to first present in a hospital not adequately equipped to provide emergency care, including ET, and are thus subsequently referred to a better equipped hospital[11,14]. This experience often results in considerable delay in providing ET[15]. Several reports also suggest that delays of up to 24 h from the time the decision to carry out ET is made and when treatment is received by the affected infant in the same hospital are not uncommon[6,14,15], compared to the estimated 4-6 h in developed countries[16]. Such delays are likely to account for the high incidence of bilirubin-induced neurological dysfunctions (ABE and kernicterus) and the associated devastating consequences in many LMICs[15,17,18]. This paper, therefore, sets out to identify commonly reported facility-based challenges in providing timely and effective ET in hospitals designated for such an emergency procedure in LMICs.
BILIRUBIN METABOLISM AND NEUROTOXICITY
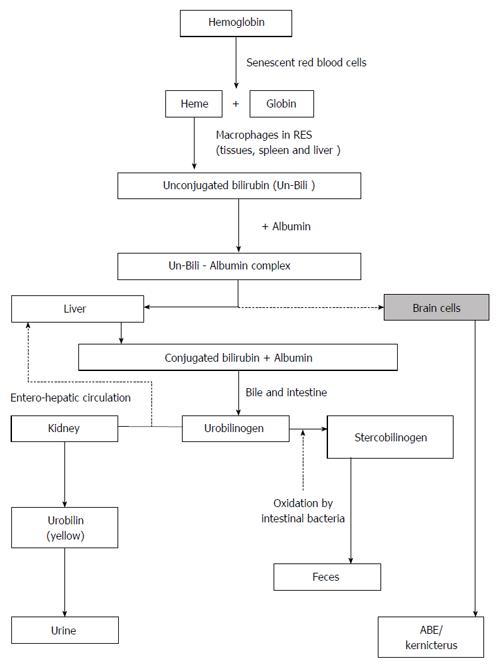
The metabolism of bilirubin has been well described in the literature[19-21]. Essentially, bilirubin production is a normal process of human physiology and begins from the degradation of heme from senescent red blood cells (Figure 1). Once produced, bilirubin is conjugated in the liver with glucuronic acid to form bilirubin glucuronide. Conjugated bilirubin is then conveyed across the canalicular membrane through the biliary tree to the intestinal lumen for excretion. Newborns, especially premature infants, have an immature bilirubin conjugation and excretion system. As a result, they have limited ability to conjugate bilirubin and excrete unconjugated bilirubin readily. These limitations account for an imbalance between bilirubin production and elimination. In effect, neonatal jaundice occurs when the rate at which bilirubin is produced exceeds the rate of elimination, reflecting the total bilirubin load in the body after birth, to become visible in the skin as yellow pigment. In full-term infants, serum bilirubin concentrations, known as physiologic jaundice, peak at 5 to 10 mg/dL in the first three days of life and decline thereafter to values commonly found in adults of approximately 1 mg/dL. However, in a few infants, serum bilirubin concentrations may become pathologic and exceed 17 mg/dL, which is indicative of a disorder that requires treatment. Total bilirubin levels beyond 17 mg/dL, especially in infants with predisposing hemolytic conditions, may lead to the movement of unconjugated bilirubin into brain cells to cause acute bilirubin encephalopathy. Continued exposure to free bilirubin may lead to irreversible damage or chronic bilirubin encephalopathy. Timely intensive phototherapy and ET can arrest this progression and prevent or minimize bilirubin-induced mortality and long-term neurologic morbidity.
Figure 1 Metabolic pathway of bilirubin neurotoxicity.
ABE: Acute bilirubin encephalopathy.
PATHWAY TO ET AND POTENTIAL CHALLENGES IN LMICs
The facilities and techniques for undertaking ET in LMICs have been well described in the literature[4,8]. The clinical criteria for initiating ET have also been discussed in greater detail elsewhere[8,22]. Typically, regardless of the total plasma/serum bilirubin (TSB) level, a “crash-cart approach” (initiation of immediate intensive phototherapy and fluid supplementation, followed by ET) is recommended for infants with early signs and symptoms of intermediate/advanced ABE (lethargy, hypotonia, poor feeding, seizures, opisthotonos, and impaired level of consciousness) with or without evidence of neurotoxicity risk factors (prematurity, isoimmune hemolytic disease, G6PD deficiency, asphyxia, sepsis, acidosis, and hypoalbuminemia). It is also worth noting that the clinical diagnosis of hemolytic jaundice remains a challenge owing to the lack of advanced tests like end-tidal carbon monoxide (ETCO), eosin-5-maleimide flow cytometry to identify red blood cell membrane defects, and next-generation sequencing of relevant genes for mutations and polymorphisms[23].
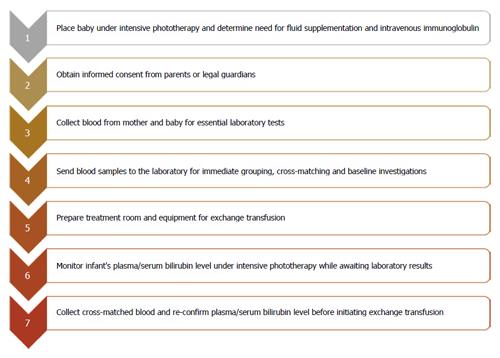
Studies describing the process from when the decision to conduct ET has been made and the actual execution of ET systematically were surprisingly rare from our literature review[4,9,24,25]. We therefore also relied on our practice experience spanning over three decades in providing newborn care in a LMIC. For example, from 2012 to 2014, approximately 120 ETs were conducted annually in our hospital, Massey Street Children’s Hospital in Lagos, which is the oldest children’s hospital in Nigeria[26]. Typically, in most clinical settings, once the need for ET has been established by the resident physician and the consultant, the typical steps to ET can be summarized as shown in Figure 2. The delays that may be encountered at any of these stages are described as follows:
Figure 2 Sequence of events and sources of potential delays following the decision to initiate exchange transfusion.
Providing intensive phototherapy preparatory to ET
Effective phototherapy has been shown to reduce the need for ET in several studies[27-31]. An effective phototherapy device should produce specific blue-light wavelengths (peak emission: 450 ± 20 nm), preferably in a narrow bandwidth to about 80% of an infant’s body surface area[32]. The light source may be fluorescent tubes, halogen lamps, or light emitting diodes. Whatever the light-source, conventional phototherapy should have an irradiance of at least 8-10 μW/cm2 per nanometer, and intensive phototherapy should have an irradiance of ≥ 30 μW/cm2 per nanometer (from either a single or multiple phototherapy units). The lack of effective phototherapy in many hospitals has been reported in several studies[33-36]. In one survey from Nigeria, for example, the vast majority (94%) of 63 phototherapy devices tested in twelve referral-level hospitals delivered irradiance of ≤ 10 μW/cm2 per nanometer and none were ≥ 30 μW/cm2 per nanometer[35].
Ineffective phototherapy is frequently attributed to erratic power supply, inadequate skin exposure (due to overcrowding from multiple infants being placed under a single device), sub-optimal irradiance levels, and poor device maintenance. A lack of intensive phototherapy during the waiting period for ET often results in a high incidence of kernicterus prior to ET and ultimately compromises the effectiveness of ET[11]. It is therefore not surprising to find adverse neurodevelopmental outcomes post-ET[17,18,37,38]. To ensure effective phototherapy, it is essential that the devices are properly monitored, regularly maintained, and that the staff are well trained to provide the best possible care for the affected infants preparatory to ET. The potential use of filtered sunlight phototherapy is currently being piloted and holds promise in tropical LMICs where effective conventional electric blue-light phototherapy devices cannot be routinely assured[39,40].
The administration of intravenous fluid supplementation should be considered for infants with evidence of dehydration, especially as a result of late presentation. This intervention has been found to decrease the need for ET by up to 70% without any long-term adverse effects[4,41]. Similarly, the use of intravenous immunoglobulin may be helpful in reducing the need for ET in infants with isoimmune hemolytic jaundice[4,42].
Obtaining informed consent and blood samples
Information on grouping and cross-matching, as well as baseline investigations such as full blood count, sodium, potassium, calcium, TSB, magnesium and glucose, are required before initiating ET. Ethical considerations forbid blood or blood product transfusion without informed consent. However, delay in getting informed consent because the mother is not available, (due to death, critical illness, or being in another hospital) or the person with parental right is unavailable is not uncommon[6]. Delay may also be encountered in trying to convince parents who are reluctant to give consent on religious grounds[11]. Additionally, the mother’s blood may not be available in time, owing to critical illness or the mother being admitted to another hospital. Difficulties may also be encountered where the mother is unavailable due to premature death. These potential sources of delay should be anticipated and addressed appropriately. It is important that prenatal maternal education be considered, especially in settings where religious beliefs are likely to delay consent for ET.
Transportation of blood samples to and collection of cross-matched blood from the laboratory
The volume of requested blood will depend on the decision for a single (estimated blood volume × baby’s weight in kilograms) or double volume (estimated blood volume × 2 × baby’s weight in kilograms) ET. Given the wide prevalence of G6PD deficiency in many LMICs, it is not uncommon for centers to have a standing rule for double-volume ET that removes 85% of the infant’s red blood cells with up to 50% TSB decline and a potential rebound to two-thirds pre-exchange level, effectively removing one-third pre-exchange TSB level[4]. However, failure to request the right amount of blood is not unusual and often results in a delay or wastage. In fact, it is more common to find clinicians over-ordering just to be assured of the availability of sufficient blood. This often results in wastage of blood and remains a potential source of friction between clinicians and the laboratory personnel[43].
Getting blood samples to the laboratory may be challenging where the functional laboratory and blood bank are outside the immediate vicinity of the hospital, as frequently encountered in many LMICs. Laboratories are often centralized to serve diverse requirements from multiple clinical units. Information from the lab may therefore be difficult to track. Where the laboratory is accessible, hospital personnel may not be immediately available, due to shortage of staff, to collect the blood as soon as the laboratory sends information to the ward that it is ready. To facilitate efficient communication with laboratory personnel, it is important to designate somebody for this task well in advance, if possible.
Preparing room and equipment for ET
The ET room must be warm and ready with essential items for the procedure, such as IV infusion pump, arterial line pack, blood warmer, and protective goggles, as well as automated monitors for cardiac, blood pressure, oxygen saturation, and respiratory function. Emergency trolley and suction equipment with appropriate catheters should be checked, stocked, and nearby. Many of these items may not be readily available and a significant number of critical items may also have to be purchased by the infant’s family. Where there is no designated room for ET, a suitable area has to be identified and screened off for the procedure. The need for infection control and keeping the baby warm must be considered.
Timely availability of laboratory results
In most hospitals, all laboratory services are centralized, implying that requests from ET personnel, even when urgent, have to be queued on arrival with other urgent requests. Laboratories in LMICs encounter several challenges that compromise their efficiency in achieving optimal turn-around time on the various requests for special investigations. These include inadequate and not up-to-date facilities, inadequate personnel, inadequate stock of blood, and, occasionally, inadequate blood samples for the required investigations.
Screening donor blood for hepatitis and human immunodeficiency virus is standard in many LMICs, but tests for G6PD status, cytomegalovirus (CMV), and malaria are often excluded, especially in regions where malaria is endemic. This may lead to using G6PD-deficient, CMV, or malaria-packed blood for ET. The use of G6PD-deficient blood has been associated with recurrent hemolysis and rebound TSB that often leads to repeat ET[44]. In the absence of blood warmer, the added time interval required to warm blood to body temperature may also prolong waiting time. Most laboratories lack diagnostic facilities for hemolytic disorders of newborns, and this frequently delays effective treatment for the affected infants.
A shortage in the number of laboratory personnel available to perform all the necessary laboratory analysis is also an important source of delay. A laboratory scientist who is in charge of carrying out the grouping and cross matching of blood for ET may be simultaneously engaged on other benches. This situation often leads to delays in issuing out blood for ET. Additionally, if the request for cross-matching gets to the laboratory very late in the day, call personnel in charge of several benches may have to be called in for grouping and cross-matching.
Blood samples from the baby may also be insufficient. Laboratory staff often complain about very small blood samples from the baby because of the method of grouping and cross-matching. A follow-up request for more blood from the laboratory causes further delay. The choice of blood, especially when the mother’s blood is not available, may also compound the problem. In situations where the mother is dead or critically ill, the best blood for ET is fresh O Rhesus “D” negative blood, but this is very scarce. Fresh whole blood less than 48 h old and not more than five days old is preferred for ET. However, since this is unattainable in most cases, the consequence is another delay in ET[13]. All blood donors should be voluntary according to internationally laid down guidelines, but blood banks in many LMICs find it difficult to convince individuals to donate blood. The end-result is delayed ET for newborns at risk of ABE/kernicterus while the perennial problem, of insufficient blood in the blood bank, persists. If the blood group that is compatible with the newborn and the mother is not available in the blood bank, other blood banks will have to be contacted, and this may extend to days before the compatible blood unit becomes available. The packed cell volume (PCV) of the donor blood is not expected to be less than 40% for male donors and 38% for female donors. However, the lack of adequate blood supply to blood banks often accounts for the reluctance of blood banks in rejecting donors with low packed red blood cell volume. Performing ET with low PCV donor blood is sub-optimum, leading invariably to additional transfusion with packed red cells.
TSB monitoring and re-confirming need for ET
Availability of real-time TSB measurement is imperative, but seldom achieved due to of the lack of a functional side laboratory with bilirubinometers in many neonatal intensive-care units. As a result, TSB monitoring still has to rely on sending blood samples to the main designated hospital laboratory for analysis. Even when intensive phototherapy is provided, the need for ET may be contingent on several factors, including accurate knowledge of the risk status of the infant and the presence of hemolytic disease. Where ET is successfully avoided as a result of the provision of effective phototherapy, the result is often unutilized blood from the blood bank. While this pattern is desirable and unavoidable, it has the impact of depleting the blood bank and causing unnecessary delay in meeting future requirements for ET. It is important to be alert to the likelihood of TSB rebound after otherwise successful intensive phototherapy, especially in infants with hemolytic jaundice. Lack of close monitoring of the affected infants may result in initially withholding ET, only for it to be later required. Failure to recognize the possibility of declining TSB level following intensive phototherapy coincident with the clinical onset of kernicterus could also be a source of potential delay[45]. It is important to view such a decline as a prognostic sign for neurologic dysfunction, rather than a sign of clinical improvement, before or after phototherapy.
The ET procedure itself seeks to remove or reduce circulating antibody-coated red blood cells and/or products of hemolysis in various immune or non-immune hemolytic anemias and other red cell enzyme deficiencies. This is accomplished by repeatedly exchanging small samples (5-10 mL/kg) of blood via an arterial catheter and replacing simultaneously with fresh donor blood providing fresh albumin with binding sites for bilirubin by continuous infusion into a peripheral or central vein. The procedure can typically last between 2 to 4 h depending on the choice between single or double volume ET.
Limited skill by clinicians can result in further delays. For example, inability to cannulate the umbilical vein and leakage of blood between the catheter and umbilical vein may unduly prolong the procedure. Difficulties may also be encountered in withdrawing blood in spite of the apparently successfully introduction of an umbilical catheter[46].
OTHER CONSIDERATIONS AND WAY FORWARD
Post-ET monitoring is necessary because of the likelihood of repeat ET after a rebound of high TSB level due to unrecognized hemolytic disease, with potential secondary delays[28,30,44]. Not all attending clinicians in emergency situations are skillful in providing ET, even where facilities are available, and this may result in delays in getting a suitable individual when all preparations have been made. In settings where ET is infrequent, lack of expertise may be a source of delay, especially when referral to another hospital becomes imperative[14]. Lack of a clearly-defined protocol or failure to adhere to an existing protocol is likely to cause delay as a result of communication gaps among team members. Where ET protocol requires the express approval of a consultant before execution by attending junior physicians, this may result in more potential delays. When more than one infant requires urgent ET and resources are limited, identifying and prioritizing the infant(s) most at-risk of kernicterus may also inevitably result in delay for some infants. Additionally, inadequate support staff may be a source of delay in providing seamless communication with the laboratory and/or a skilled assistant for the procedure. In some settings, patients may be required to bear the costs of the laboratory investigations requested by the attending physicians, especially in private hospitals[47,48]. Inability to meet such expenses is also a potential source of delay in providing timely ET[49].
The nature and scope of these delays are likely to vary within and across LMICs. Perhaps the overarching implication of these challenges is the impetus to avoid ET as much as possible by facilitating early presentation and timely provision of effective/intensive phototherapy, as well as investment in functional, readily accessible, and appropriately staffed laboratories in all hospitals that offer emergency care for newborns. Side laboratory with facilities for real-time bilirubin measurements should be made available in all neonatal units. Education of mothers and caregivers on the value of timely presentation and intervention in preventing bilirubin-induced mortality and long-term neurodevelopmental disorders should be routinely offered during antenatal visits. There is also a need for better communication and understanding between clinicians and laboratory personnel, especially with regards to the challenge of minimizing wastage of blood due to over-ordering[43].
While the focus of this review is primarily to serve the needs of clinicians in LMICs, the emerging and rising profile of global child health makes the topic also relevant to clinicians in the developed world.