Peer-review started: August 22, 2018
First decision: October 14, 2018
Revised: November 15, 2018
Accepted: January 8, 2019
Article in press: January 8, 2019
Published online: January 15, 2019
Processing time: 146 Days and 23.6 Hours
The regeneration of peripheral nerves comprises complicated steps involving a set of cellular and molecular events in distal nerve stumps with axonal sprouting and remyelination. Stem cell isolation and expansion for peripheral nerve repair (PNR) can be achieved using a wide diversity of prenatal and adult tissues, such as bone marrow or brain tissues. The ability to obtain stem cells for cell-based therapy (CBT) is limited due to donor site morbidity and the invasive nature of the harvesting process. Dental pulp stem cells (DPSCs) can be relatively and simply isolated from the dental pulps of permanent teeth, extracted for surgical or orthodontic reasons. DPSCs are of neural crest origin with an outstanding ability to differentiate into multiple cell lineages. They have better potential to differentiate into neural and glial cells than other stem cell sources through the expression and secretion of certain markers and a range of neurotropic factors; thus, they should be considered a good choice for PNR using CBT. In addition, these cells have paracrine effects through the secretion of neurotrophic growth factors and extracellular vesicles, which can enhance axonal growth and remyelination by decreasing the number of dying cells and activating local inhabitant stem cell populations, thereby revitalizing dormant or blocked cells, modulating the immune system and regulating inflammatory responses. The use of DPSC-derived secretomes holds great promise for controllable and manageable therapy for peripheral nerve injury. In this review, up-to-date information about the neurotrophic and neurogenic properties of DPSCs and their secretomes is provided.
Core tip: The distinct developmental pathway of dental pulp stem cells (DPSCs) from neural crest cells results in a cell type that can be participate in neural tissue regeneration. The efficacy of using DPSCs for peripheral nerve repair (PNR) is strongly influenced by boosting trophic factors that promote axonal growth and regeneration and provide direct and indirect protection against cell death. Recently, encouraging results from different studies indicate that DPSC secretomes have reparative and protective properties comparable with their cellular counterparts in PNR. The use of DPSC secretomes as a safe and possibly more valuable substitute for cell-based therapy approaches is a novel therapeutic perspective.
- Citation: Sultan N, Amin LE, Zaher AR, Scheven BA, Grawish ME. Dental pulp stem cells: Novel cell-based and cell-free therapy for peripheral nerve repair. World J Stomatol 2019; 7(1): 1-19
- URL: https://www.wjgnet.com/2218-6263/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5321/wjs.v7.i1.1
Structurally, the nervous system comprises two main components, the peripheral nervous system (PNS) and the central nervous system (CNS). The CNS involves the spinal cord and brain and acts as the motor output and center for all sensory perception. The low regenerative capacity of the CNS makes injury to these regions permanent because the damaged neurons undergo degenerative cell death and are not substituted[1]. The PNS includes the sensory nerves, motor nerves and ganglia outside the spinal cord and brain. The peripheral nerves transfer signals throughout the body and the spinal cord. These signals are sent to the brain and provide sensory information when a reflex response is provided[2]. The proper function and maintenance of peripheral nerves are primarily controlled by cells other than neurons, specifically, Schwann cells (SCs) that surround the nerves and release important trophic factors, such as nerve growth factor (NGF) which is important during the process of nerve repair and is responsible for proliferation, growth regulation and survival of target neurons[3].
Peripheral nerve injury (PNI) may result in the loss of motor function, sensory function, or both. Such injury leads to neurapraxia, axonotmesis or neurotmesis and may occur as a result of acute compression, trauma, iatrogenic induction during surgical procedures, diabetes or other health conditions such as Guillain-Barre syndrome. Patients with PNI encounter several challenges, ranging from mild discomfort to long-term functional defect. During end-organ denervation, reinnervation can occur in two ways: through collateral branching of unbroken axons or through regeneration of the damaged axon[4]. Collateral branching occurs in cases where 20%-30% of the axon' cells within a nerve are damaged and is considered the main recovery mechanism. In injuries disturbing more than 90% of the axon' cells within a nerve, axonal regeneration is the primary method of recovery[5]. To accomplish full recovery, the axon undertakes three main processes: clearing of the distal stump or Wallerian degeneration, axonal regeneration, and end-organ reinnervation. Poor functional consequences usually experienced by patients with PNI result from the failure of any of these processes[6].
SCs play fundamental roles in the maintenance and survival of healthy axons and in axonal regeneration, and they transfer essential molecules across the axons. They produce a variety of neurotrophic factors that interact with tyrosine kinases and other receptors and modify the neuron gene expression profile to enhance regeneration[7]. Within healthy nerves, NGF has a low expression level, but it is upregulated in SCs during injury. This factor promotes proliferation and growth of certain target neurons[6]. Many neurotrophic factors have been discovered in neurons and in SCs, and they function to improve cell survival through apoptosis prevention mechanisms and enhancing regeneration processes[8]. Furthermore, studies have evaluated SCs for engraftment and myelination of injured nerves in animal models, providing a foundation for axonal regeneration and functional recovery[9]. However, SC harvesting is limited for multiple reasons; among them the need to sacrifice one or more functional nerves for SC isolation with subsequent neuroma formation, donor site morbidity and loss of sensation, thereby leading to a demand for other cell sources[10].
Adult stem cells are considered multipotent undifferentiated cells that have the ability to self-renew and differentiate into several cell lineages. These adult cells have been successfully isolated from different tissues, such as neural, bone, retina, skin and dental tissue. Adult mesenchymal stem cells (MSCs) can be isolated from a wide range of tissues, such as bone marrow (BMSCs), adipose tissue (AMSCs) and dental tissues. Postnatally, dental stem cells can be isolated from different dental tissues for example from dental pulp tissue (DPSCs), human exfoliated primary teeth (SHED)[11], the tissue of the apical papilla (SCAP)[12] and periodontal ligament surrounding the roots of teeth (PDLSC)[13]. The ability of MSCs to restore injured tissue is generally related to their chemokine surface receptors which enable them to migrate toward injured tissue under the influence of growth factors or chemokines secreted by the damaged target organ. MSCs have immune-modulatory, anti-inflammatory and multilineage differentiation potential [14,15].
MSCs and their secretomes, which include paracrine factors secreted into the extracellular matrix, have been extensively studied for their potential to promote nerve regeneration. In 2013, Teixeira et al[16], suggested that MSCs isolated from Wharton jelly of the umbilical cord, bone marrow or adipose tissue are capable of nerve repair due to their differentiation ability and multipotency. In addition to their differentiation potential, MSCs are capable of secreting neuroregulatory factors that promote neurogenesis and survival of glial cells and neurons. However, the transplanted cells cannot survive long; therefore, most of the attention has been focused on the bioactive molecules secreted by MSCs, including growth factors and cytokines. These factors are secreted into the extracellular matrix which is actively involved in the guidance, regulation and control of tissue homeostasis, development and regeneration[17].
DPSCs are a neural crest derived cells that possess MSC properties[18]. DPSCs are easily harvested and isolated from extracted teeth. Storage and cryopreservation of DPSCs are essential steps for banking of these cells for future application and use[19,20]. DPSCs have the ability to differentiate into multiple cell lineages with the potential to differentiate into neural cells. Surprisingly, DPSCs in an undifferentiated state can express neural markers, such as S100, β-III-tubulin and NGFR p75[21]. Under appropriate conditions, DPSCs have been successfully differentiated into SCs and acquire both neuronal morphology and function. It was found that DPSCs express characteristic SC markers, such as laminin and CD104[22]. Moreover, they promote neurite outgrowth of trigeminal neurons and rescue motor neurons in spinal cord injury models and exhibit typical SC interactions with neurons, such as neuritis myelination. Furthermore, they are able to secrete a range of neurotrophic factors including vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF) and NGF. These properties together with their availability make DPSCs an auspicious tool for cell-based therapy (CBT) for PNI[23].
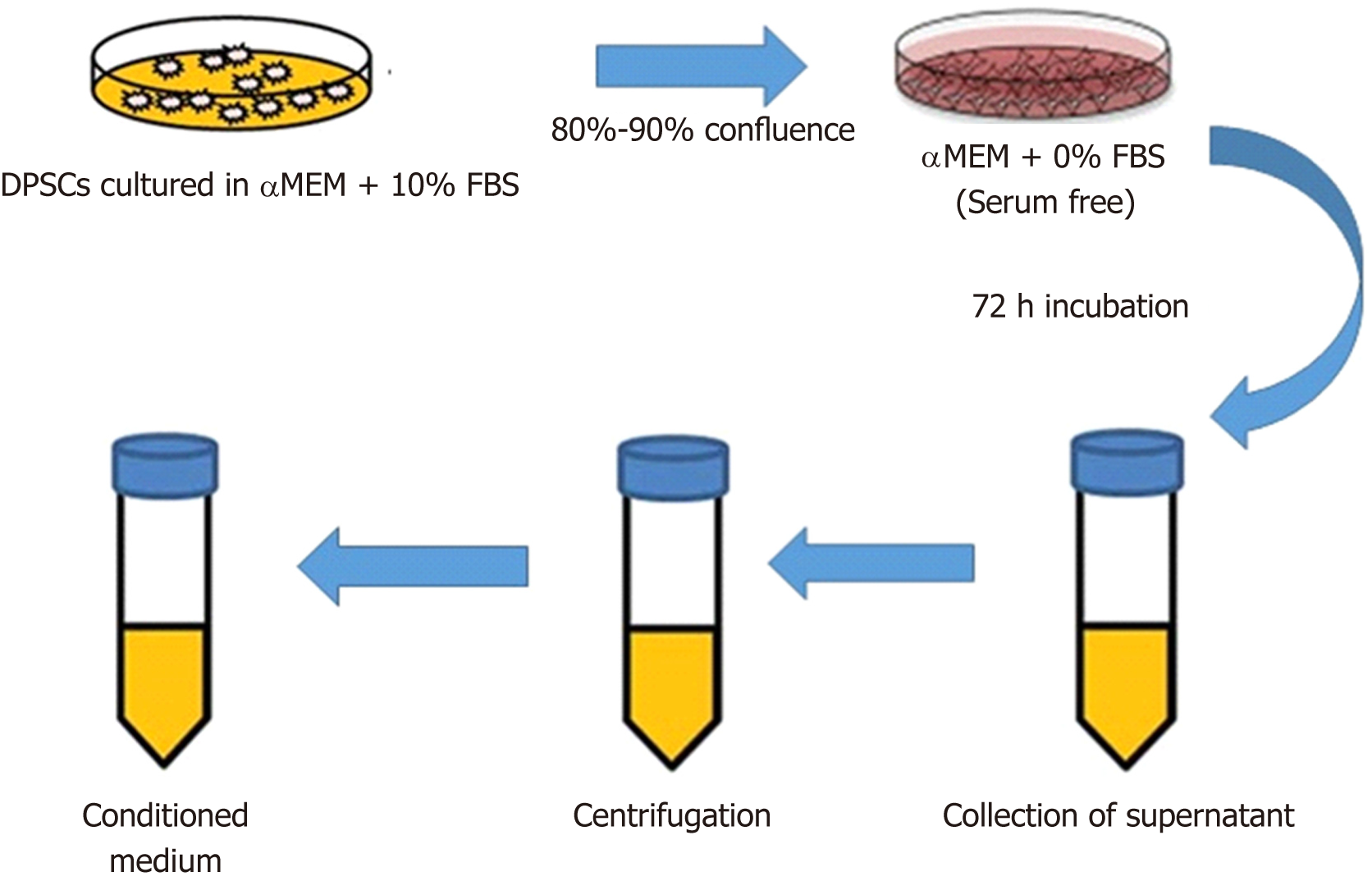
DPSC-conditioned medium (CM), i.e., the cell culture supernatant (Figure 1), was previously regarded as waste that contains cell debris, but now, it recognized that CM contains the regenerative ambience of secretomes. Immune-regulation, anti-fibrotic, and anti-apoptotic properties; the ability to stimulate angiogenesis and neurogenesis; and a variety of biological activities have been attributed to secretomes[24-28]. In this review, we will discuss therapeutic application of DPSCs and their secretomes for peripheral nerve repair (PNR). Where relevant, comparisons between CBT with DPSCs and cell-free therapy (CFT) using secretomes will be noted. We will briefly summarize the neurogenic and neurotrophic properties of DPSCs and their secretomes and then summarize the main differences between CBT and CFT.
The PNS has an intrinsic repair and regeneration capability; however, this ability is restricted and depends on many factors, such as the mechanism of injury, age of the patient and especially the proximity of the injury to the nerve cell body[29]. PNI accompanied by sensory disturbance or pain in the orofacial region is considered a major clinical challenge and may lead to permanent disability[30]. Distal nerve stump denervation for prolonged periods, especially in large gaps, is often accompanied by a decrease in the number of SCs; therefore, the outcomes following PNI remain poor. The cell bodies of axons after nerve transection start to swell in an attempt to address the increased metabolic demand necessary for regeneration and some nerve cells will shift to neuronal cell apoptosis. Then, SCs, which are considered the principal glial cells of the PNS, start to proliferate, convert to a phagocytic phenotype and begin to attract circulating macrophages to the site of damage. These important steps serve to remove axonal and myelin debris from the distal stump in an attempt to prepare the injury site for the regenerating axon. At the same time, SCs start to secrete growth factors, which create favorable environmental conditions for nerve regrowth toward the target tissue[31,32]. Thus, nerve regeneration is a complicated process that is regulated through the interplay of complex cell signaling processes. These signaling processes direct SC migration and axonal outgrowth to bridge the nerve gap between two ends of transected peripheral nerve stumps.
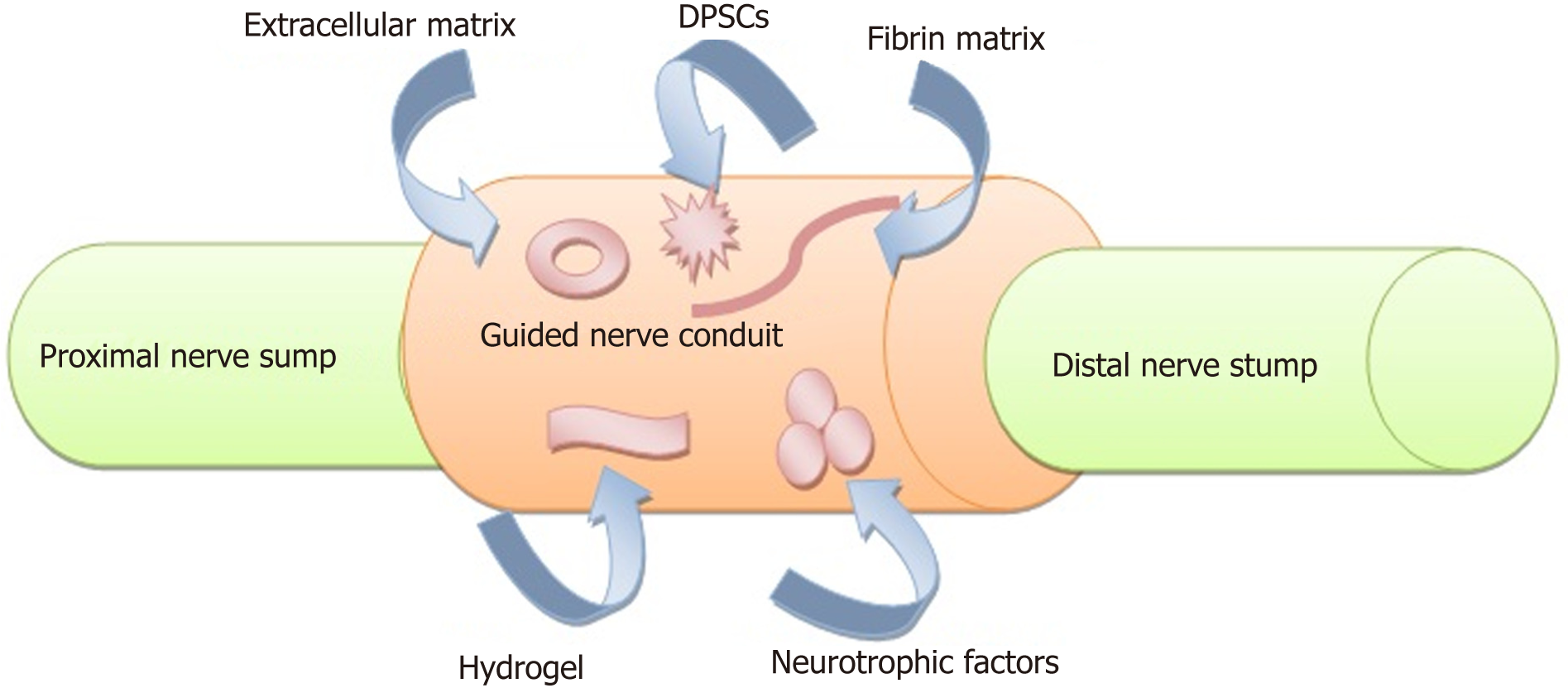
Any interruption during this process might lead to down regulation of the growth factors and loss of axonal regeneration[33,34]. On the other side, the denervated target organ will undergo a fatigue of trophic factors, atrophy of muscle fibers and apoptosis of satellite cells[35]. Treatment modalities such as braces or splints, electrical stimulators, physical and occupational therapy and exercise are insufficient[36]. Surgical treatments of PNI with autologous nerve grafts is considered the gold standard; however, this treatment has the disadvantage of donor site morbidity, and available grafts may be limited in length and there is a potential for neuroma formation. Today, complete recovery is rare despite all the types of available treatment modalities, and these limitations increase the demand for alternative modalities (Figure 2) for nerve reconstruction[37-40]. Currently, meticulous microsurgical repair is the best choice, especially with the use of tensionless epineural sutures. Nevertheless, in the presence of a wide nerve gap at which end-to-end suturing is impossible, autologous nerve grafting should be considered the optimal solution[41]. Nerve injuries should be repaired as quickly as possible because any delay in the repair process may have a significant detrimental effect on sensory and motor recovery[42].
Stem cells, undifferentiated cells that are capable of differentiating into specific and specialized cell types, can be allocated into several categories: (1) Embryonic stem cells (ESCs) obtained from the embryoblast of a blastocyst; (2) induced pluripotent stem cells (iPSCs) created directly from adult cells reprogrammed to become embryonic-like pluripotent cells; and (3) adult stem cells, including hematopoietic stem cells and MSCs. ESCs, iPSCs, MSCs and neural stem cells (NSCs) have been studied in vitro and in vivo for their ability to aid in nerve repair. ESCs promoted the repair of a 10-mm gap in rat sciatic nerve in a histological, electrophysiological and molecular study[43]. SCs-like precursors were generated from ESCs in models of PNR in vitro, and these cells expressed myelin protein[44]. When NSCs were seeded in chitosan, they give results comparable with autografts in repairing a 10-mm nerve gap[45]. Additionally, iPSCs can be used to efficiently repair a 10-mm nerve gap and produce functional neural crest cells[46].
Despite the fact that ESCs have unique characteristics, such as an unlimited quantity and pluripotency, the clinical application of these cells has been restricted by safety problems, such as immunogenic reactions, ethical concerns, low efficiency, tumorigenicity and inadequate availability[47]. Compared with ESCs, the use of MSCs in regenerative medicine is accompanied by fewer ethical issues, considering the risk of teratoma formation, the sourcing of the cells and undesired cell differentiation. The ability of MSCs to differentiate into different cell lineages with specific and predetermined environmental conditions and to exhibit immunosuppressive properties has enabled their fruitful transplantation into a well-matched donor[14,15]. Allogeneic MSCs are comparable to autologous MSCs from nonhuman primates and were not rejected[48].
Recently, in CBT for PNI, emphasis has been given to creating satisfactory environmental conditions for axon regeneration. The goal is to increase SCs number and activity because they are the orchestrators of PNR and to prevent their senescence[34]. Achieving sufficient numbers of autologous SCs for culture requires healthy nerve scarification and extended periods of expansion and purification that subsequently delay the repair process. Prolonged denervation leads to loss of SC-mediated axonal support and apoptosis of the cell body in the peripheral nerve. Therefore, autologous transplantation of SCs is considered impossible[49,50]. Optimal stem cell criteria for PNR should include that cells are easily accessible, rapidly expanded in vitro, easily integrate into host tissue and are capable of survival in vivo[51].
The potential of MSCs to regenerate injured tissue is basically related to three mechanisms: “homing”, which refers to the ability of stem cells to migrate to the target organ due to chemical gradients[52]; their ability to self-renew and the potential for multilineage differentiation; and a paracrine mechanism via secretion of a broad array of bioactive factors[53,54]. In tissue engineering and/or regenerative medicine applications, engrafted stem cells are susceptible to ischemic attack, and this may lead to limited paracrine secretion and function and poor survival of grafted cells[55]. Recent research has shown that the paracrine effects of MSC secretomes are an important factor in CBT and may have a direct or indirect influence on the surrounding microenvironments[56-58].
Alge et al[59], compared DPSCs and BMSCs and reported that DPSCs were superior in all examined properties, including proliferation, differentiation and mineralization potential. Additionally, Mead et al[60], confirmed that DPSCs exhibit significantly more neuroprotective and neuritogenic effects on retinal ganglion cells than BMSCs or AMSCs; they also concluded that DPSCs secreted higher amounts of numerous growth factors, such as containing BDNF, NGF, VEGF and GDNF, which play pivotal roles in neuroprotection and neuritogenesis. They concluded that MSCs have distinctive neurotrophic gene expression profiles, but specifically, DPSCs expressed prostaglandin E2 receptor (EP2) at higher levels than both BMSCs and AMSCs. EPs have a role in the release and synthesis of neurotrophins from different cell types.
DPSCs share a common origin with peripheral nerve glial progenitor cells[61]. This feature makes these cells a very interesting choice for PNR. Nestin expression in DPSCs is well documented and suggests the ability of these cells to differentiate into neuronal lineages because they originate from neural crest cells. DPSCs express neuronal markers, such as neurofilament (NF), ΒIII-tubulin and glial fibrillary acidic protein (GFAP). This indicates that there is a great similarity in membrane properties between DPSCs and neuronal cells[62,63]. Moreover, when DPSCs were grown on non-adherent culture plastic, they reorganized from a uniform cell monolayer and switched to a more quiescent state distinguished by the presence of spheroid structures similar to neurospheres, which stained positive for nestin[64-67]. Therefore, DPSCs are a heterogeneous population ranging from neuroblast-like to fibroblast-like cells[68,69]. The high expression of neurotransmitter receptors and neural markers by DPSCs suggest that these cells vigorously respond to neural environmental signals, promoting re-establishment of functional nerve conductivity[70-72].
Martens et al[23], confirmed the potential of DPSCs to differentiate into SCs in vitro that efficiently myelinated dorsal root ganglion neurons, a result confirmed by an ultrastructural TEM analysis. Regarding the significant role that SCs play in axonal peripheral nerve regeneration and protection and the obstacles in their maintenance and harvesting, the use of DPSC-derived autologous SCs may be considered an important step in designing new treatments for PNI. One of the DPSC-derived neurotrophic factors is GDNF which reverses the symptoms associated with neuropathic pain and exerts a powerful analgesic effect[73]. Small fiber neuropathy was treated using a small molecule modulator of ligand-induced GFRα/RET receptor signaling through a topical application[74].
A literature search was performed in September 2018 in the PubMed database. The following keywords were used: dental pulp stem cells[Title/Abstract] AND nerve repair[Title/Abstract], and the search retrieved 6 results and searching with keywords dental pulp stem cells[Title/Abstract] AND nerve regeneration[Title/Abstract] retrieved 12 results. Exclusion of duplicate and review articles yielded 15 results[14,23,60,75-86]. Overall, 8 studies[14,75-81] were found that tested in-vivo and in-vitro application of DPSCs (Table 1), 2 studies[82,83] were conducted in-vivo with animal models (Table 2) and 5 studies[23,60,84-86] only performed cell culture tests (Table 3). The source of stem cells, target tissue, study model, and objective and outcomes of the retrieved studies were included and are described in the abovementioned tables.
| Author (publication year) | Source of stem cells | Target tissue | Study model | Objective | Outcome | |
| Carnevale et al[14], 2018 | In-vivo | Human STRO-1+ /c-Kit+ /CD34+DPSCs expressing P75NTR, nestin and SOX-10 | Sciatic nerve defect | Animal rat model | To demonstrate the ability of human STRO-1+ /c-Kit+ /CD34+ DPSCs expressing P75NTR, nestin and SOX-10 to promote axonal regeneration. | The cells promoted regeneration and functional recovery of sciatic nerve defects after injury. |
| In-vitro | Human STRO-1+ /c-Kit+ /CD34+DPSCs expressing P75NTR, nestin and SOX-10 | To differentiate into SC-like cells | In-vitro culturing of DPSCs and their differentiation to SCs | To demonstrate the ability of Human STRO-1+ /c-Kit+ /CD34+ DPSCs expressing P75NTR, nestin and SOX-10 to differentiate into SC-like cells. | Under appropriate conditions, the cells differentiated into SC-like cells | |
| Kolar et al[75], 2017 | In-vivo | Adult rat SCs; Human SCAP, DPSCs and PDLSC | 10 mm nerve gap defect in a rat sciatic nerve | Sciatic nerve injury model | To demonstrate the ability of human SCAP, DPSCs and PDLSC to promote axonal regeneration using nerve guidance conduit of 14 mm length. | All the stem cell types significantly enhanced axon regeneration after two weeks. SCAP are the optimal dental stem cell type for peripheral nerve repair. |
| In-vitro | CM from unstimulated or stimulated human SCAP, DPSCs and PDLSC | Differentiated human neuroblastoma SH-SY5Y cell line | In-vitro neurite outgrowth assay | To examine the biological activity of the conditioned medium for unstimulated and stimulated human SCAP, DPSC and PDLSC. | Quantification of the neurite outgrowth showed that unstimulated and stimulated human SCAP, DPSCs and PDLSC increased both the percentage of cells producing neurites and the total neurite outgrowth length. | |
| Omi et al[76], 2017 | In-vivo | DPSCs isolated from the incisor teeth of 6-wk-old male rats | Sciatic nerve; Sensory nerve fibers; Sural nerves | Streptozotocin-induced diabetic rats. | Investigated whether the transplantation of DPSCs ameliorated long-term diabetic polyneuropathy in streptozotocin-induced diabetic rats. | Significant reductions in the sciatic motor/sensory nerve conduction velocity, increases in the current perception threshold, and decreases in capillary density in skeletal muscles and intra-epidermal nerve fiber density. Sural nerve morphometrical analysis revealed that the transplantation of DPSCs significantly increased the myelin thickness. |
| In-vitro | DPSCs isolated from the incisor teeth of 6-wk-old male rats | Dorsal root ganglion neuron were cultured for use in neurite outgrowth with DPSC-CM; Immortalized adult Fischer rat SCs were cultured with DPSC-CM | In-vitro neurite outgrowth assay; Cell viability assay | Evaluation of neurite outgrowth. Analysis of myelin-related protein formation in immortalized adult Fischer rat SCs. | DPSCs-CM promoted the neurite outgrowth of dorsal root ganglion neurons. Increased the viability and myelin-related protein expression of SCs. | |
| Sanen et al[77], 2017 | In-vivo | SCs derived from differentiated human DPSCs | 15-mm rat sciatic nerve defects | Sciatic nerve injury model | Evaluated the performance of SCs derived from differentiated human DPSCs in a rat model of PNI. | Immunohistochemistry and ultrastructural analysis revealed in-growing neurites, myelinated nerve fibres and blood vessels along the construct. |
| In-vitro | SCs derived from differentiated human DPSCs | Human microvascular endothelial cell line (HMEC-1) | Alamar Blue cell proliferation assay; Transwell migration assay; Tube formation assay | Investigated the neuroregenerative and the proangiogenic capacities of SCs derived from differentiated human DPSCs. | The endothelial cell line HMEC-1 had proliferated significantly more in the presence of conditioned medium from human DPSCs and differentiated human DPSCs compared with those in control medium. | |
| Hei et al[78], 2016 | In-vivo | Schwann-like cells were derived from human DPSCs; Human DPSCs | 3 mm - wide crush injury was inflicted at a distance of approximately 10 mm from the mental foramen | Male Sprague-Dawley rats crush-injury site | To investigate the effect of Schwann-like cells combined with pulsed electromagnetic field on peripheral nerve regeneration. | Schwann-like cells, human DPSCs with or without pulsed electromagnetic field, and pulsed electromagnetic field only improved peripheral nerve regeneration. |
| In-vitro | Schwann-like cells were derived from human DPSCs; Human DPSCs | Schwann Cells | Cell culture dishes | To demonstrate the ability of hDPSCs to differentiate into Schwann - like cells and demonstrate glial character with expression of CD104, S100, GFAP, laminin and p75NTR. | Successful morphological differentiation of hDPSCs toward Schwann - like cells. | |
| Yamamoto et al[79], 2016 | In-vivo | Human mobilized DPSCs | 5-mm gap of the left sciatic nerve | Rat sciatic nerve defect model | To investigate the effects of human mobilized DPSC transplantation on peripheral nerve regeneration using 9-mm collagen conduit. | Human mobilized DPSCs promote axon regeneration through trophic functions, acting on SCs and promote angiogenesis. |
| In-vitro | CM of human mobilized DPSCs | Rat SCs (RT4-D6P2T) | Migration, proliferation, and anti-apoptotic assays | To investigate the trophic effects of mobilized human DPSCs on proliferation, migration and anti-apoptosis in SCs | The human mobilized DPSCs-CM significantly enhanced proliferation and migratory activity and decreased apoptosis of RT4-D6P2T cells. | |
| Askari et al[80], 2014 | In-vivo | Human DPSCs transfected with a tetracycline-inducible system expressing oligodendrocyte lineage transcription factor 2 gene | Sciatic nerve demyelination experiment | Mouse model of local sciatic demyelination damage by lysolecithin | To investigate if the tetracycline-regulated expression of oligodendrocyte lineage transcription factor 2 gene transfected in human DPSCs can lead to mouse sciatic nerve regeneration upon transplantation. | Human DPSCs-derived oligodendrocyte progenitor cells have relevant therapeutic potential in the animal model of sciatic nerve injury. |
| In-vitro | Human DPSCs | Oligodendrocyte | In-vitro plasmid construct and transfection | DPSCs were transfected with oligodendrocyte transcription factor 2 which play important role in differentiation of DPSCs to oligodendrocyte progenitor cells. | Exogenous expression of the oligodendrocyte lineage transcription factor 2 gene by a tetracycline-regulated system could be used as an efficient way to induce the differentiation of DPSCs into functional oligodendrocytes. | |
| Dai et al[81], 2013 | In-vivo | SCs, AMSCs, DPSCs, and the combination of SCs with AMSCs or DPSCs | 15-mm-long critical gap defect of rat sciatic nerve | Sciatic nerve injury model | To test their efficacy in repairing PNI 17-mm nerve conduit. | Co-culture of SCs with AMSCs or DPSCs in a conduit promoted peripheral nerve regeneration over a critical gap defect. |
| In-vitro | SCs, AMSCs, DPSCs, and the combination of SCs with AMSCs or DPSCs | Neuronal cells | RT-PCR analysis of the coculture in-vitro | To verify if the combination of cells led to synergistic neurotrophic effects NGF, BDNF, and GDNF. | Results confirmed the synergistic NGF production from the co-culture of SCs and ASCs. |
| Author | Publication year | Source of stem cells | Target nerves | Study model | Objective | Outcome |
| Ullah et al[82], 2017 | 2017 | Human DPSCs; Differentiated neuronal cells from DPSCs | 5-mm gap sciatic nerve transection | Animal rat model | To evaluate the in-vivo peripheral nerve regeneration potential of human DPSCs and differentiated neuronal cells from DPSCs. | In-vivo transplantation of the undifferentiated hDPSCs could exhibit sufficient and excellent peripheral nerve regeneration potential. |
| Spyridopoulos et al[83], 2015 | 2015 | DPSCs isolated from second lateral incisor pigs | Transected fifth and sixth intercostal nerves | Animal pig model | Examined the potential of DPSCs for peripheral nerve regeneration, using biodegradable collagen conduits. | The nerves where DPSCs were injected exhibited morphological and functional recovery. |
| Author | Publication year | Source of stem cells | Target tissues | Objective | Outcome |
| Geng et al[84], 2017 | 2017 | Human DPSCs | Differentiation of hDPSCs. | To demonstrate the differentiating ability of resveratrol on DPSCs. | Resveratrol induced DPSCs differentiation into neuroprogenitor cells. DPSCs might be an important cell population for neurological disease treatment. |
| Hafner et al[85], 2017 | 2017 | Human DPSCs | Spider dragline silk fibers | To evaluating adhesion and alignment of dental pulp stem cells to a spider silk substrate for tissue engineering applications. | Natural drawn spider silk acted as an effective substrate for cellular adhesion and alignment of DPSCs and could be used in neural differentiation applications. |
| Chang et al[86], 2014 | 2014 | Human DPSCs | Medium preparation for the induction of spinal motor neuronal differentiation; Medium preparation for the induction of dopaminergic neuronal differentiation | To evaluate the efficacy of dopaminergic and motor neuronal inductive media on transdifferentiation of human DPSCs (hDPSCs) into neuron-like cells. | Human DPSCs-derived dopaminergic and spinal motor neuron cells after induction expressed a higher density of neuron cell markers than those before induction. |
| Mead et al[60], 2014 | 2014 | Human DPSC, human BMSCs human AMSCs | Axotomised adult rat retinal ganglion cells | To evaluate the therapeutic potential for neurodegenerative conditions of retinal ganglion cells. | Human DPSCs promoted significant multi-factorial paracrine-mediated retinal ganglion cell survival and neurite outgrowth compared with Human BMSCs/Human AMSCs. |
| Martens et al[23], 2014 | 2014 | Human DPSCs | Dorsal root ganglia | Evaluated the differentiation potential of human DPSCs toward SCs, together with their functional capacity with regard to myelination and support of neurite outgrowth. | Human DPSCs are able to undergo SCs differentiation and support neural outgrowth. |
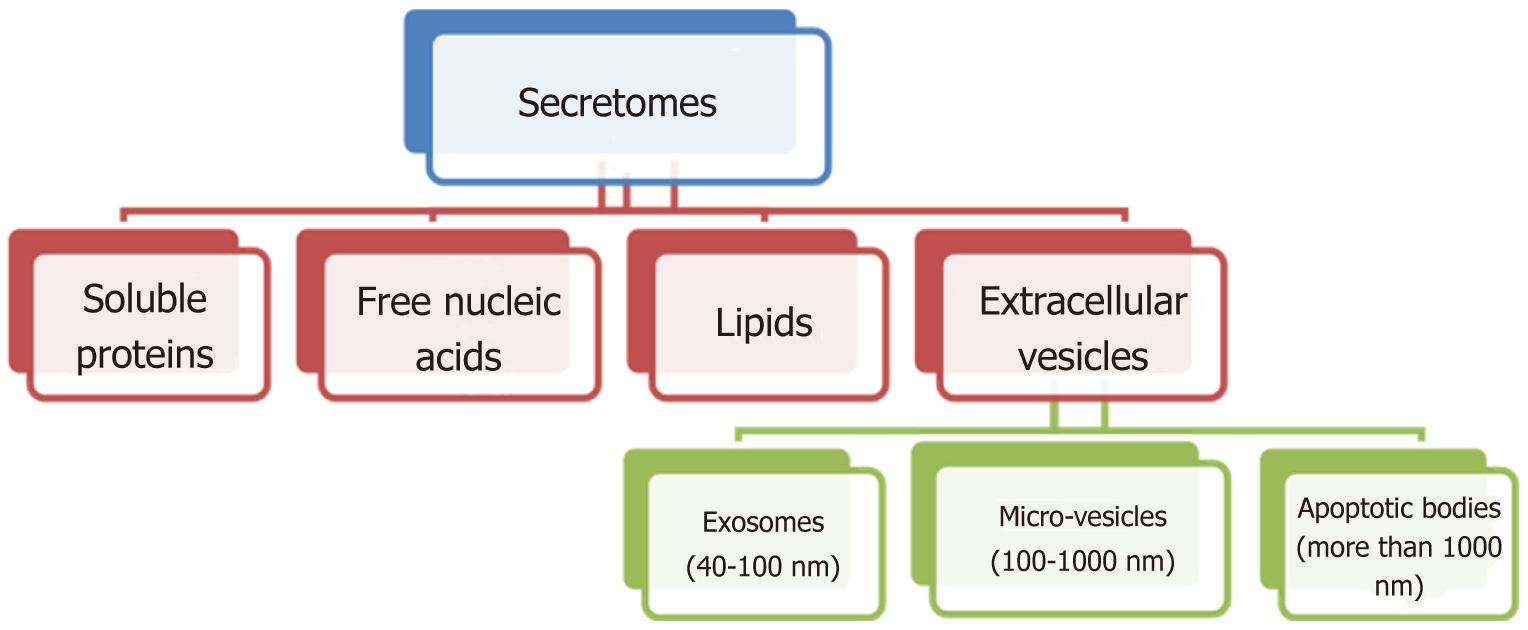
The paracrine effect of MSCs is mediated through their secretomes to promote the repair processes[87,88]. Secretomes contain a broad range of bioactive soluble factors with anti-apoptotic, anti-fibrotic, angiogenetic, chemo-attractive and immunomodulation properties, and these components include free nucleic acids, soluble proteins, extracellular vesicles and lipids. Extracellular vesicles can be subdivided into microvesicles (MVs) and exosomes (Figure 3)[89].
Exosomes, MVs and apoptotic bodies are secreted vesicles that can be distinguished from each other by morphology and size. Exosome sizes range from 40-100 nm whereas MV sizes range from 100-1000 nm and apoptotic bodies are more than 1000 nm in size[90]. Exosomes are usually derived from multivesicular bodies, while MVs are formed by plasma membrane budding and anti-apoptotic bodies via blebbing of the plasma membrane of dying cells[91]. Exosomes and MVs usually contain proteins, lipids, mRNAs and microRNAs which are important in cell-cell communication, Exosomes exert their action by delivering their contents directly into cells without the need for specific receptor expression. Bilayer membrane encapsulation provides protected environmental conditions that allow them to travel within the body without degradation[92]. Mead et al[93], highlighted more than 40 surplus miRNAs in MSCs compared with fibroblast exosomes, suggesting that the combination of miRNAs may responsible for exosome mediated neuroprotection[94].
Nakano et al[95] designed genetically engineered MSC secretomes and reported that these cells were able to secrete hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), transforming growth factor-β (TGF-β), and VEGF, which are closely related to neurite outgrowth and neuronal survival in vitro. After MSCs transplantation, they were able to secrete NGF, NT-3, GDNF and a high level of BDNF, leading to in vivo axonal growth. Comparable results were also reported by Neuhuber et al[96], who found that MSC secretomes could promote axonal growth and recovery of neuron function owing to the presence of neurotrophic factors[97,98].
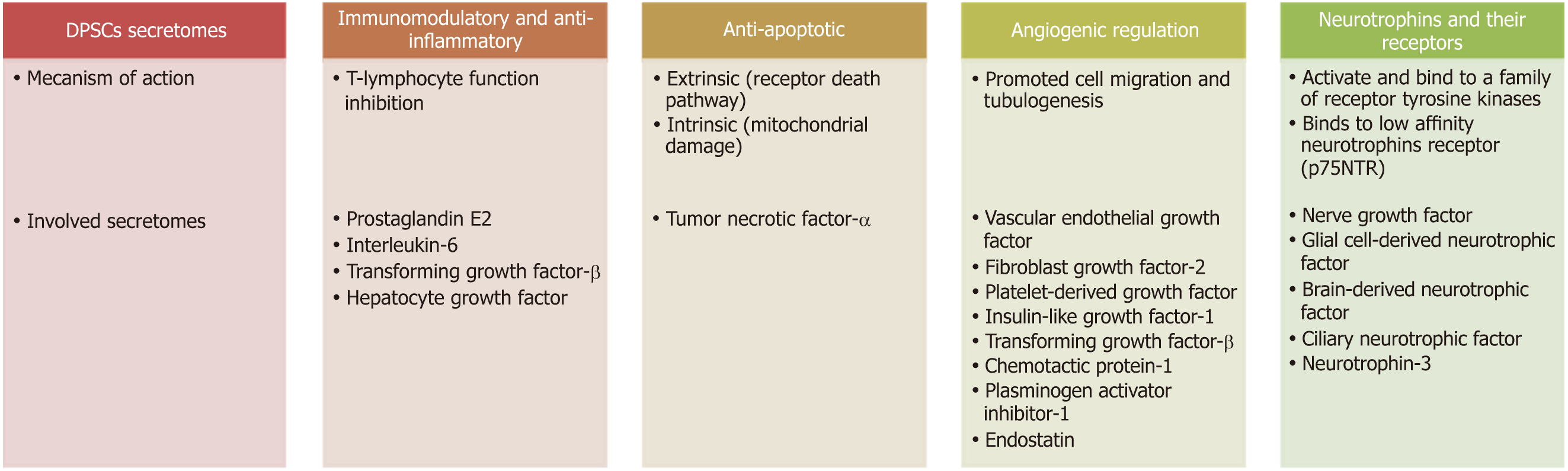
DPSC secretomes include immunomodulatory, anti-inflammatory, anti-apoptotic and angiogenic regulatory and neurotrophic factors (Figure 4).
The immunomodulatory function of DPSCs is mediated through T-lymphocyte function inhibition and this occurs through the action of prostaglandin E2, interleukin-6 (IL-6), TGF-β and HGF secreted from DPSCs[99,100]. Other studies have implicated HGF and TGF-β as DPSCs mediators due to their anti-proliferative effect on T cells. These results were further supported by as study showing upregulation of TGF-β and HGF transcripts during MSCs/T cell cocultures[101].
Neuronal apoptosis or programmed cell death is an important process after nerve injury. Basically, there are two apoptosis pathways, the extrinsic or death receptor pathway, which is activated by tumor necrosis factor-α (TNF-α) overexpression; and the intrinsic pathway, which occurs through mitochondrial damage. DPSCs can prevent TNF-α overexpression and maintain the level of Bcl-xl, thus blocking the extrinsic and intrinsic mechanisms and subsequently decreasing neuronal apoptosis[102].
Angiogenesis is regulated by both inhibitory and stimulatory molecules[103]. Several studies have shown that MSC/DPSCs are able to express angiogenic factors, such as FGF-2, VEGF, IGF-1, PDGF and TGF-β. Additionally, numerous anti-angiogenic factors have been detected in cultures of DPSCs such as plasminogen activator inhibitor-1, chemotactic protein-1 and endostatin[104-106]. Moreover, DPSCs are capable of inducing in vitro migration of endothelial cells and in vivo formation of blood vessels and exhibit a higher angiogenic capability than BMSCs/AMSCs[99]. Application of DPSC secretomes to endothelial cells enhanced tubulogenesis and cell migration, demonstrating the paracrine and pro-angiogenic effect of the cell secretomes[107]. Angiogenesis appears to be of prime importance in nerve repair because newly formed blood vessels act as tracks/guiding paths for SCs to cross the bridge gap taking regrowing axons with them[108].
Neurotrophins are a group of proteinaceous substances that induce the development, function and survival of neurons. Neurotrophins activate and bind to a family of receptor tyrosine kinases (TRKs). NT-3 binds to TrkC, BDNF to TrkB and NGF to TrkA. Binding of these receptors to their factors presents a survival signal to neurons. Another receptor, named p75NTR, also binds to neurotrophins but with lower affinity[109]. P75NTR is an indispensable receptor that works in coordination with the TRK family, transducing the signals from NGF, BDNF and NT-3 to regulate a broad array of processes essential to maintenance and development of the nervous system[110].
DPSCs from both rats and humans release neurotrophins, including NGF, GDNF, BDNF and CNTF. Neurotrophins enhance neurite guidance, promote growth of neurons both in vivo and in vitro, stimulate rescue survival of neurons and induce neurogenesis at the site of injury. They recruit endogenous cells to differentiate into specific cell types necessary for nerve regeneration at the site of damage and stimulate the endogenous cells to secrete neurotrophic factors, promoting tissue regeneration. In animal models and in spinal cord injury, the production of neurotrophins by DPSCs has been shown to rescue motor neurons and mediate the survival of sensory and dopaminergic neurons in addition to the survival of trigeminal ganglia and sympathetic neurons[22].
NGF involved in differentiation and survival of sympathetic and sensory neurons. Its role in neural development has been comprehensively studied. Zhang et al[111] found that low NGF concentrations are effective in promoting stem cell proliferation. NGF was also shown to guide the migration of SCs in the PNS, and this is mediated through p75NTR[112,113]. BDNF is also one of the neurotrophins that is intensely involved in numerous developmental events in the nervous system, including proliferation, differentiation, migration, apoptosis and survival[114]. This factor helps in neuronal survival and encourages growth and differentiation of new neurons[115]. In the developing visual cortex, exogenous use of BDNF promotes the complexity of pyramidal neurons, with an increase in dendritic length in a layer-specific manner, suggesting that BDNF modulates a specific form in dendritic growth in addition to enhancing neuronal growth[116,117].
In experimental animal models, NT-3 has been verified to promote regeneration of injured axons, enhance neurite outgrowth and improve axon function. Cells overexpressing NT-3 migrated more and displayed longer neurites in vitro and in vivo than other cells[118,119]. CNTF has powerful therapeutic effects on nerve apoptosis, neuro-inflammation and neuronal proliferation[120,121]. Additionally, GDNF potently promotes the survival of many types of neurons. The most prominent feature of GDNF is its ability to support the survival of motor and dopaminergic neurons[22].
As discussed above, neurotrophic factors released by DPSCs/MSCs may act as modulators of neural differentiation and survival[122-124]. Individual use of these trophic factors or even combinations of them appears to be unsuccessful and less efficient at enhancing regeneration than the full secretomes[95,125]. The possibility of repairing injured tissue with secretomes rather than cell therapy introduces a new era for therapeutic application of secretomes in regenerative medicine[126]. Mead et al[60] confirmed the role of neurotrophins in neuroprotection and neuritogenesis using the fusion protein Fc-NTFR to block neurotrophin receptor sites and examine the mechanism of DPSCs/BMSCs/AMSCs-mediated neuroprotection and neuritogenesis when cocultured with retinal ganglion cells. The DPSCs neuroprotective effect was considerably decreased after addition of Fc-NTFR, confirming the major role of neurotrophins (GDNF, BDNF, NGF, VEGF, NT-3 and PDGF) in neuroprotection and neuritogenesis.
The main challenge with CBT is how to maintain cell viability and function after in vivo implantation because in vivo conditions are very different from well-controlled in vitro cultivation conditions. Factors such as tissue collagen density, blood supply and scar formation potential greatly affects the fate of transplanted cells[127]. For instance, the spreading of fibroblasts initially increases with the increase in collagen density, but beyond a specific limit, the relationship is reversed. At a high collagen density, the attempt of a cell to spread maximally is restricted by the availability of collagen binding sites and consequently, cells exert a maximal force to tightly bind with the few available sites[128]. Moreover, the molecular environment in injured tissue enhances apoptotic cell death; therefore, massive death of the transplanted cells ocurres. This is due to the elevated level of oxidative stress, mediated by reactive oxygen species, in the injured tissue, which triggers cell apoptosis[129].
Bork et al[130] reported that DNA methylation and epigenetic changes occurred in replicative senescence upon long term cultivation. Lack of differentiation potential, cell enlargement and eventual growth arrest occurs in replicative senescence. A similar methylation pattern was observed in MSCs from older donors. These findings support evidence showing that aging and replicative senescence represent a developmental program and are not only caused by accumulation of cellular and molecular defects, and thus, long-term culture and aging might be regulated by similar mechanisms. At the same time, the long-term cultivation process leads to stress-induced senescence changes due to high oxygen content and the artificial in vitro environment. This brings about a decrease in the self-renewal potential of cells, and when such cells are implanted in the host, poor growth, cell survival and paracrine effect outcomes result. Therefore, there are still many hurdles before stem CBT can be adopted for PNI and it becomes decisive to determine a strategy to overcome difficulties and the problems related to cell transplantation[131].
In the field of regenerative medicine, the use of CFT has been widely studied. The use of the secretomes overcomes a number of safety concerns that include tumorigenicity, emboli formation and immune compatibility. Secretomes can be stored for a long period without application of a potentially toxic cryopreservative and without loss of product potency[132,133]. An important feature of exosomes, which are part of the secretome, is that they are encapsulated, providing protection to their contents against in vivo degradation, thus possibly avoiding obstacles associated with soluble small molecules, such as transcription factors, cytokines, growth factors and RNAs, that are quickly degraded[134]. Moreover, exosomes can act as liposomes and can pass through the blood-brain barrier, making them potentially suitable for treatment of neurological disorders[135].
To sum up, secretomes contain soluble growth factors and cytokines related to protection, repair, regeneration, immunomodulation, cell proliferation, cell communication and other important functions[136]. Use of DPSCs/MSCs secretomes has several advantages, and we can avoid concerns relating to cell transplantation. Recently, a comparative study demonstrated that treatment with secretomes induced a long-lasting effect with a disease-modifying profile similar to that shown by stem cells. Wakayama et al[137] illustrated the effect of DPSCs and their secretomes on acute lung injury; they examined the persistence and localization of DPSCs transplanted to an injured lung. It was found that the survival of DPSCs was less than 1% one week after transplantation and the therapeutic benefit of DPSCs and DPSCs secretomes was similar two weeks after transplantation. Therefore, the researchers concluded that the therapeutic effect of DPSCs was likely mediated via paracrine signaling that remains for an extended period of time even after cell disappearance. This study opens new possibilities for treatment using a cell-free approach that is able to retain the benefits of cell therapy without the inherent difficulties of CBT[138].
It is essential to consider the possible adverse effects of the therapeutic potential of exosomes against their future application. It has been stated that miRNAs that are carried by exosomes might induce cancer or tumor formation[139]. In addition, exosomes may be associated with a number of neurologic diseases related to old age, such as Parkinson’s and Alzheimer’s diseases[140]. Moreover, the ideal timeframe for injection/addition of exosomes to exploit their benefits and whether a single application dose is sufficient or if daily, weekly or monthly doses are necessary are still unknown. Additionally, different epitopes are expressed on the surface of exosomes released from the same cells, indicating the presence of exosome subtypes, which merits further research[141]. Further in-depth research is guaranteed to lead to an improved understanding of exosomes and their interference with unknown secreted factors.
DPSCs secretomes are a promising strategy for CBT and CFT. They can be easily isolated, purified and stored, thus avoiding complications associated with cell therapy, such as unwanted proliferation/differentiation and development of ectopic tissue. To appraise the role of the surrounding microenvironment in the biological response of DPSCs, preconditioning may be helpful to obtain tailor-made secretomes. Preconditioning achieved by subjecting cells to hypoxia, drug treatment, and specific growth factor/cytokines may help in obtaining an optimal secretome profile. The ideal timeframe for injection/addition of the secretomes to exploit their benefits is still unknown, as well as whether a single application dose is sufficient or if daily, weekly or monthly doses are necessary. Additionally, different surface epitopes are expressed on exosomes from the same cells, indicating the presence of exosome subtypes, which merits further research.
Manuscript source: Invited manuscript
Specialty type: Dentistry, oral surgery and medicine
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fatkhudinov T, Hassan AI, Pixley JS S- Editor: Cui LJ L- Editor: A E- Editor: Bian YN
| 1. | Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L. Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys. 2014;68:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Farrar FC, White D, Darnell L. Pharmacologic Interventions for Pain Management. Crit Care Nurs Clin North Am. 2017;29:427-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Aguayo AJ, Peyronnard JM, Bray GM. A quantitative ultrastructural study of regeneration from isolated proximal stumps of transected unmyelinated nerves. J Neuropathol Exp Neurol. 1973;32:256-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Panthi S, Gautam K. Roles of nitric oxide and ethyl pyruvate after peripheral nerve injury. Inflamm Regen. 2017;37:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lunn ER, Brown MC, Perry VH. The pattern of axonal degeneration in the peripheral nervous system varies with different types of lesion. Neuroscience. 1990;35:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Hall S. Axonal regeneration through acellular muscle grafts. J Anat. 1997;190:57-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990;110:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 263] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia. 2001;34:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Arora V, Arora P, Munshi AK. Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent. 2009;33:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 785] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 13. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2469] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 14. | Carnevale G, Pisciotta A, Riccio M, Bertoni L, De Biasi S, Gibellini L, Zordani A, Cavallini GM, La Sala GB, Bruzzesi G, Ferrari A, Cossarizza A, de Pol A. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J Tissue Eng Regen Med. 2018;12:e774-e785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1078] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 16. | Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871-3882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 17. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 839] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 18. | Graham A, Begbie J, McGonnell I. Significance of the cranial neural crest. Dev Dyn. 2004;229:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3307] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 20. | Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig. 2013;17:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Li Z, Wei H, Deng L, Cong X, Chen X. Expression and secretion of interleukin-1β, tumour necrosis factor-α and interleukin-10 by hypoxia- and serum-deprivation-stimulated mesenchymal stem cells. FEBS J. 2010;277:3688-3698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 27. | Lin W, Li M, Li Y, Sun X, Li X, Yang F, Huang Y, Wang X. Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci. 2014;35:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA. 2016;113:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 348] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 29. | Ramachandran S, Midha R. Editorial. Outcomes of facial nerve repair using nerve grafts applied immediately following nerve discontinuity in skull base surgery. J Neurosurg. 2018;128:627-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Zuniga JR. Trigeminal ganglion cell response to mental nerve transection and repair in the rat. J Oral Maxillofac Surg. 1999;57:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 32. | Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 383] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 33. | Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31:5325-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World J Stem Cells. 2015;7:11-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Heck CS, Davis HL. Effect of denervation and nerve extract on ultrastructure of muscle. Exp Neurol. 1988;100:139-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Rosén A, Tardast A, Shi TJ. How Far Have We Come in the Field of Nerve Regeneration After Trigeminal Nerve Injury? Curr Oral Health Rep. 2016;3:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Bagheri SC, Meyer RA. Management of mandibular nerve injuries from dental implants. Atlas Oral Maxillofac Surg Clin North Am. 2011;19:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Siemionow M, Brzezicki G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Moore AM, MacEwan M, Santosa KB, Chenard KE, Ray WZ, Hunter DA, Mackinnon SE, Johnson PJ. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve. 2011;44:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 40. | Pindrik J, Belzberg AJ. Peripheral nerve surgery: primer for the imagers. Neuroimaging Clin N Am. 2014;24:193-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Millesi H. Bridging defects: autologous nerve grafts. Acta Neurochir Suppl. 2007;100:37-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Jivan S, Kumar N, Wiberg M, Kay S. The influence of pre-surgical delay on functional outcome after reconstruction of brachial plexus injuries. J Plast Reconstr Aesthet Surg. 2009;62:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, Yu SP. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26:1356-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Ziegler L, Grigoryan S, Yang IH, Thakor NV, Goldstein RS. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev. 2011;7:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Guo BF, Dong MM. Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg. 2009;140:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Ikeda M, Uemura T, Takamatsu K, Okada M, Kazuki K, Tabata Y, Ikada Y, Nakamura H. Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J Biomed Mater Res A. 2014;102:1370-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Brunt KR, Weisel RD, Li RK. Stem cells and regenerative medicine - future perspectives. Can J Physiol Pharmacol. 2012;90:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 306] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 488] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 50. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15:3876-3885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 319] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 51. | Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908-3913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 679] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 52. | Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 53. | Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Zhao X, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Transpl Int. 2006;19:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1614] [Cited by in RCA: 1530] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 55. | Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12-d26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 596] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 56. | Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82-83:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 57. | Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 58. | Ando Y, Matsubara K, Ishikawa J, Fujio M, Shohara R, Hibi H, Ueda M, Yamamoto A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone. 2014;61:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, Gabriel Chu TM, Goebel WS. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 61. | Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 62. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 63. | Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015;14:243-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 64. | Ibarretxe G, Crende O, Aurrekoetxea M, García-Murga V, Etxaniz J, Unda F. Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration. Stem Cells Int. 2012;2012:103503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Bonnamain V, Thinard R, Sergent-Tanguy S, Huet P, Bienvenu G, Naveilhan P, Farges JC, Alliot-Licht B. Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol. 2013;4:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Xiao L, Tsutsui T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: stem cells in the core. J Cell Biochem. 2013;114:2624-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Gervois P, Struys T, Hilkens P, Bronckaers A, Ratajczak J, Politis C, Brône B, Lambrichts I, Martens W. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. 2015;24:296-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Fujita S, Hideshima K, Ikeda T. Nestin expression in odontoblasts and odontogenic ectomesenchymal tissue of odontogenic tumours. J Clin Pathol. 2006;59:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Ichikawa H, Kim HJ, Shuprisha A, Shikano T, Tsumura M, Shibukawa Y, Tazaki M. Voltage-dependent sodium channels and calcium-activated potassium channels in human odontoblasts in vitro. J Endod. 2012;38:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27:2229-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Varga G, Gerber G. Mesenchymal stem cells of dental origin as promising tools for neuroregeneration. Stem Cell Res Ther. 2014;5:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Király M, Porcsalmy B, Pataki A, Kádár K, Jelitai M, Molnár B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 384] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 74. | Hedstrom KL, Murtie JC, Albers K, Calcutt NA, Corfas G. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRα/RET receptor signaling. Proc Natl Acad Sci U S A. 2014;111:2325-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7:12605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 76. | Omi M, Hata M, Nakamura N, Miyabe M, Ozawa S, Nukada H, Tsukamoto M, Sango K, Himeno T, Kamiya H, Nakamura J, Takebe J, Matsubara T, Naruse K. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res Ther. 2017;8:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Sanen K, Martens W, Georgiou M, Ameloot M, Lambrichts I, Phillips J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J Tissue Eng Regen Med. 2017;11:3362-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Hei WH, Kim S, Park JC, Seo YK, Kim SM, Jahng JW, Lee JH. Schwann-like cells differentiated from human dental pulp stem cells combined with a pulsed electromagnetic field can improve peripheral nerve regeneration. Bioelectromagnetics. 2016;37:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 79. | Yamamoto T, Osako Y, Ito M, Murakami M, Hayashi Y, Horibe H, Iohara K, Takeuchi N, Okui N, Hirata H, Nakayama H, Kurita K, Nakashima M. Trophic Effects of Dental Pulp Stem Cells on Schwann Cells in Peripheral Nerve Regeneration. Cell Transplant. 2016;25:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 80. | Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S. Human Dental Pulp Stem Cells Differentiate into Oligodendrocyte Progenitors Using the Expression of Olig2 Transcription Factor. Cells Tissues Organs. 2014;200:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Dai LG, Huang GS, Hsu SH. Sciatic nerve regeneration by cocultured Schwann cells and stem cells on microporous nerve conduits. Cell Transplant. 2013;22:2029-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 82. | Ullah I, Park JM, Kang YH, Byun JH, Kim DG, Kim JH, Kang DH, Rho GJ, Park BW. Transplantation of Human Dental Pulp-Derived Stem Cells or Differentiated Neuronal Cells from Human Dental Pulp-Derived Stem Cells Identically Enhances Regeneration of the Injured Peripheral Nerve. Stem Cells Dev. 2017;26:1247-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Spyridopoulos T, Lambropoulou M, Pagonopoulou O, Birbilis T, Tsaroucha AK, Kouzi-Koliakou K, Botaitis S, Deftereou TE, Gaitanidis A, Pitiakoudis M. Regenerated Nerve Defects with a Nerve Conduit Containing Dental Pulp Stem Cells in Pigs: An Immunohistochemical and Electrophysiological Evaluation. J Reconstr Microsurg. 2015;31:516-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Geng YW, Zhang Z, Liu MY, Hu WP. Differentiation of human dental pulp stem cells into neuronal by resveratrol. Cell Biol Int. 2017;41:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Hafner K, Montag D, Maeser H, Peng C, Marcotte WR Jr, Dean D, Kennedy MS. Evaluating adhesion and alignment of dental pulp stem cells to a spider silk substrate for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2017;81:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Chang CC, Chang KC, Tsai SJ, Chang HH, Lin CP. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formos Med Assoc. 2014;113:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 87. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1162] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 88. | Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 89. | Skalnikova H, Motlik J, Gadher SJ, Kovarova H. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics. 2011;11:691-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 90. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6036] [Article Influence: 503.0] [Reference Citation Analysis (0)] |
| 91. | György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667-2688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1350] [Cited by in RCA: 1611] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 92. | Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 348] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 93. | Mead B, Tomarev S. Retinal ganglion cell neuroprotection by growth factors and exosomes: lessons from mesenchymal stem cells. Neural Regen Res. 2018;13:228-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, Wu D, Zhang ZG. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol Neurobiol. 2017;54:2659-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 95. | Nakano N, Nakai Y, Seo TB, Yamada Y, Ohno T, Yamanaka A, Nagai Y, Fukushima M, Suzuki Y, Nakatani T, Ide C. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett. 2010;483:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 230] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 97. | Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 98. | Park HW, Lim MJ, Jung H, Lee SP, Paik KS, Chang MS. Human mesenchymal stem cell-derived Schwann cell-like cells exhibit neurotrophic effects, via distinct growth factor production, in a model of spinal cord injury. Glia. 2010;58:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 99. | Ishizaka R, Hayashi Y, Iohara K, Sugiyama M, Murakami M, Yamamoto T, Fukuta O, Nakashima M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials. 2013;34:1888-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 100. | Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, Colic M. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 2011;20:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 101. | Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217-226. [PubMed] |
| 102. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7556] [Cited by in RCA: 9378] [Article Influence: 521.0] [Reference Citation Analysis (0)] |
| 103. | Sieveking DP, Ng MK. Cell therapies for therapeutic angiogenesis: back to the bench. Vasc Med. 2009;14:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 104. | Tran-Hung L, Laurent P, Camps J, About I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol. 2008;53:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 105. | Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res. 2006;85:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 107. | Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, Wolfs E, Bronckaers A, Hilkens P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J Endod. 2017;43:S12-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015;162:1127-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 638] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 109. | Gao X, Daugherty RL, Tourtellotte WG. Regulation of low affinity neurotrophin receptor (p75(NTR)) by early growth response (Egr) transcriptional regulators. Mol Cell Neurosci. 2007;36:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 110. | Blöchl A, Blöchl R. A cell-biological model of p75NTR signaling. J Neurochem. 2007;102:289-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Zhang L, Jiang H, Hu Z. Concentration-dependent effect of nerve growth factor on cell fate determination of neural progenitors. Stem Cells Dev. 2011;20:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci U S A. 1994;91:2795-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 254] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 113. | Bose KS, Sarma RH. Delineation of the intimate details of the backbone conformation of pyridine nucleotide coenzymes in aqueous solution. Biochem Biophys Res Commun. 1975;66:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 796] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 115. | Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3285] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 116. | Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J, Elzinga BM, Voshaar RC. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 117. | Pillai A, Bruno D, Sarreal AS, Hernando RT, Saint-Louis LA, Nierenberg J, Ginsberg SD, Pomara N, Mehta PD, Zetterberg H, Blennow K, Buckley PF. Plasma BDNF levels vary in relation to body weight in females. PLoS One. 2012;7:e39358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Lu H, Li M, Song T, Qian Y, Xiao X, Chen X, Zhang P, Feng X, Parker T, Liu Y. Retrovirus delivered neurotrophin-3 promotes survival, proliferation and neuronal differentiation of human fetal neural stem cells in vitro. Brain Res Bull. 2008;77:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Kamei N, Tanaka N, Oishi Y, Hamasaki T, Nakanishi K, Sakai N, Ochi M. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine (Phila Pa 1976). 2007;32:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 120. | Fargali S, Sadahiro M, Jiang C, Frick AL, Indall T, Cogliani V, Welagen J, Lin WJ, Salton SR. Role of neurotrophins in the development and function of neural circuits that regulate energy homeostasis. J Mol Neurosci. 2012;48:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Pasquin S, Sharma M, Gauchat JF. Ciliary neurotrophic factor (CNTF): New facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev. 2015;26:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 122. | Agis-Balboa RC, Fischer A. Generating new neurons to circumvent your fears: the role of IGF signaling. Cell Mol Life Sci. 2014;71:21-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 123. | Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE. Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J Neuropathol Exp Neurol. 2014;73:734-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 124. | Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, Manadas B, Behie LA, Sousa N, Salgado AJ. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl Med. 2017;6:634-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 125. | Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271-2285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 126. | Dahbour S, Jamali F, Alhattab D, Al-Radaideh A, Ababneh O, Al-Ryalat N, Al-Bdour M, Hourani B, Msallam M, Rasheed M, Huneiti A, Bahou Y, Tarawneh E, Awidi A. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. 2017;23:866-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 127. | Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, Song J, Zhang Y. Strategies to Optimize Adult Stem Cell Therapy for Tissue Regeneration. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 128. | Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, Dembo M, Wong JY. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J. 2003;85:3329-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 129. | Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed Res Int. 2015;2015:617207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 130. | Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 317] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 131. | Benn SC, Woolf CJ. Adult neuron survival strategies--slamming on the brakes. Nat Rev Neurosci. 2004;5:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 132. | Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 133. | Eiró N, Sendon-Lago J, Seoane S, Bermúdez MA, Lamelas ML, Garcia-Caballero T, Schneider J, Perez-Fernandez R, Vizoso FJ. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget. 2014;5:10692-10708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 134. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8246] [Cited by in RCA: 9668] [Article Influence: 537.1] [Reference Citation Analysis (0)] |
| 135. | Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 728] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 136. | Bruno S, Collino F, Tetta C, Camussi G. Dissecting paracrine effectors for mesenchymal stem cells. Adv Biochem Eng Biotechnol. 2013;129:137-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 137. | Wakayama H, Hashimoto N, Matsushita Y, Matsubara K, Yamamoto N, Hasegawa Y, Ueda M, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. 2015;17:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 138. | Gama KB, Santos DS, Evangelista AF, Silva DN, de Alcântara AC, Dos Santos RR, Soares MBP, Villarreal CF. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018;2018:8179013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 139. | Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 884] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 140. | Tofaris GK. A Critical Assessment of Exosomes in the Pathogenesis and Stratification of Parkinson’s Disease. J Parkinsons Dis. 2017;7:569-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 141. | Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |