Copyright
©The Author(s) 2017.
World J Orthop. Feb 18, 2017; 8(2): 130-141
Published online Feb 18, 2017. doi: 10.5312/wjo.v8.i2.130
Published online Feb 18, 2017. doi: 10.5312/wjo.v8.i2.130
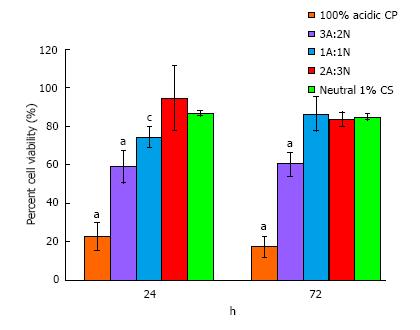
Figure 2 In vitro direct contact biocompatibility normalized to tissue culture plastic control reported as the average ± standard deviation of percent cell viability for 100% acidic chitosan paste, 3A:2N, 1A:1N, and 2A:3N chitosan/polyethylene glycol paste variations, and neutral 1% chitosan sponges after 24 and 72 h (n = 5).
(aP < 0.05 vs all at respective time point, cP < 0.05 vs 2A:3N CPP and neutral 1% CS at respective time point). CPP: Chitosan/polyethylene glycol paste.
- Citation: Rhodes CS, Alexander CM, Berretta JM, Courtney HS, Beenken KE, Smeltzer MS, Bumgardner JD, Haggard WO, Jennings JA. Evaluation of a chitosan-polyethylene glycol paste as a local antibiotic delivery device. World J Orthop 2017; 8(2): 130-141
- URL: https://www.wjgnet.com/2218-5836/full/v8/i2/130.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i2.130