Published online Feb 18, 2017. doi: 10.5312/wjo.v8.i2.115
Peer-review started: April 28, 2016
First decision: July 5, 2016
Revised: October 31, 2016
Accepted: November 21, 2016
Article in press: November 22, 2016
Published online: February 18, 2017
Processing time: 294 Days and 3 Hours
Patellar instability is a common clinical problem encountered by orthopedic surgeons specializing in the knee. For patients with chronic lateral patellar instability, the standard surgical approach is to stabilize the patella through a medial patellofemoral ligament (MPFL) reconstruction. Foreseeably, an increasing number of revision surgeries of the reconstructed MPFL will be seen in upcoming years. In this paper, the causes of failed MPFL reconstruction are analyzed: (1) incorrect surgical indication or inappropriate surgical technique/patient selection; (2) a technical error; and (3) an incorrect assessment of the concomitant risk factors for instability. An understanding of the anatomy and biomechanics of the MPFL and cautiousness with the imaging techniques while favoring clinical over radiological findings and the use of common sense to determine the adequate surgical technique for each particular case, are critical to minimizing MPFL surgery failure. Additionally, our approach to dealing with failure after primary MPFL reconstruction is also presented.
Core tip: An increasing number of revision surgeries of the reconstructed medial patellofemoral ligament (MPFL) will be seen in the foreseeable future. There are several reasons for this trend: (1) The increasing number of primary MPFL reconstructions; (2) The fact that more and more orthopedic surgeons perform this surgical technique; and (3) The high percentage of patients returning to sport after this type of surgery and thereby put the reconstructed ligament at risk. Our paper tries to answer a crucial question: What must we do to reduce the number of failed MPFL reconstructions? Furthermore, we analyze our approach to dealing with failure after MPFL reconstruction.
- Citation: Sanchis-Alfonso V, Montesinos-Berry E, Ramirez-Fuentes C, Leal-Blanquet J, Gelber PE, Monllau JC. Failed medial patellofemoral ligament reconstruction: Causes and surgical strategies. World J Orthop 2017; 8(2): 115-129
- URL: https://www.wjgnet.com/2218-5836/full/v8/i2/115.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i2.115
What is a failure of a medial patellofemoral ligament (MPFL) reconstruction? A MPFL reconstruction in patients with chronic lateral patellar instability (CLPI) fails when there is either recurrence of the instability, disabling anterior knee pain (AKP) or a combination of both. While this usually demands a revision surgery, there are some more questionable cases. It ultimately depends on the activity level and how much this instability or AKP affects a patient with the same ligament deficiency. The higher the physical requirements are the greater the disability caused by the malfunctioning MPFL. Patients with low physical requirements will tolerate instability much better and will have less instability and/or pain. In addition, standard scales (Kujala, IKDC) used to measure results in “normal” people are not practical for athletes due to their low sensitivity. Instead, functional tests that includes specific sporting gestures (cutting, pivoting, stopping, etc.)[1] should be used in this specific group of patients.
Shah et al[2], in a systematic review (meta-analysis-level of evidence II) of complications and failures associated with the MPFL reconstruction in patients with a CLPI, found that the complication rate associated with this procedure (26%) is not at all insignificant even though MPFL has a high success rate. Therefore, it is important to inform the patient of the potential risks of this surgery before the surgery. These authors also showed that instability represents 32% of all the complications (52/164) found in MPFL reconstruction[2]. This recurrence of instability may be secondary to a ruptured or elongated MPFL graft, or secondary to the failure to recognize other risk factors for instability. However, Parikh et al[3] found a slightly smaller rate of complications (16%) in a case series (level of evidence IV). Surprisingly enough, almost half of those complications resulted from technical problems or surgical errors. Ultimately, most failed MPFL reconstructions result from surgeon-dependent factors. Schneider et al[4] reported a low rate of reoperations after an isolated MPFL reconstruction, specifically 3.1% (95%CI: 1.1%-5.0%), in a systematic review and meta-analysis published in 2016. However, this study only reported on short term results. Similarly, the recurrence of instability and the persistence of apprehension was 1.2% (95%CI: 0.3%-2.1%) and 3.6%, respectively (95%CI: 0%-7.2%).
The increasing number of primary surgeries will lead to a higher number of MPFL revision surgeries in upcoming years. Schneider et al[4] showed that 84.1% (95%CI: 71.1%-97.1%) of patients return to sports after an isolated MPFL reconstruction. Thus, the return to sports puts the reconstructed ligament at risk and so its break again due to an indirect trauma to the knee.
This paper tries to answer a crucial question: What must we do to reduce the number of failed MPFL reconstruction? An approach to dealing with failure after primary MPFL reconstruction is also presented.
The first requirement for a successful MPFL reconstruction is, logically, to properly select the patient. The ideal indication of an isolated MPFL reconstruction would be a CLPI with at least two documented episodes of dislocation, and confirmation of dislocation with examination under anesthesia, in a patient with a TT-TG distance of less than 20 mm, a positive apprehension test up to 30° of knee flexion, a patellar Caton-Deschamps index of less than 1.2 and trochlear dysplasia grade A[5]. A double-bundle MPFL reconstruction is recommended given that it is associated with a lower failure rate than single bundle reconstruction[6].
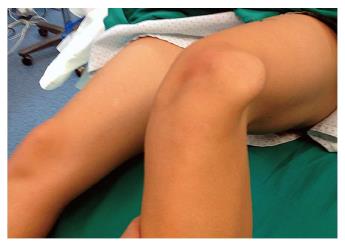
On the other hand, an MPFL reconstruction is not indicated in patients with AKP without patellar instability. Neither is it indicated for excessive lateral patellar tilt and/or lateral patellar subluxation on imaging without a history and a physical examination for CLPI. Lateral patellofemoral instability, with at least 2 documented episodes of patellar dislocations and a physical examination demonstrating patellar dislocation, is the primary indication for an MPFL reconstruction[5]. Pain and “giving out” episodes are not sufficient criteria for establishing this diagnosis. Examination under anesthesia may be necessary to confirm lateral patellar instability objectively (Figure 1). A MPFL reconstruction should not be performed if the patella cannot be laterally dislocated.
An MPFL reconstruction is not aimed at “pulling” the patella into position, but rather at stabilizing it once the patellofemoral tracking has been corrected. That is so once the patella is in an adequate position within the trochlear groove. Therefore, an isolated MPFL reconstruction is not indicated to eliminate patella J-tracking.
Finally, an isolated MPFL reconstruction should not be performed with fixed lateral patellar dislocation in knee flexion (Figure 2). In this situation, the main problem is the retraction of the extensor mechanism of the knee and a flat lateral condyle, factors that contribute to secondary MPFL insufficiency[7]. Therefore, the correct treatment for these cases would be a lateral retinaculum lengthening, lengthening of the rectus lateralis tendon and quadriceps tendon lengthening[7]. If needed, the lateral condyle may be raised. Then, an MPFL reconstruction may be performed as the final surgical step[7].
According to Parikh et al[3], 47% of the complications that occur after MPFL reconstructive surgery are related to technical errors.
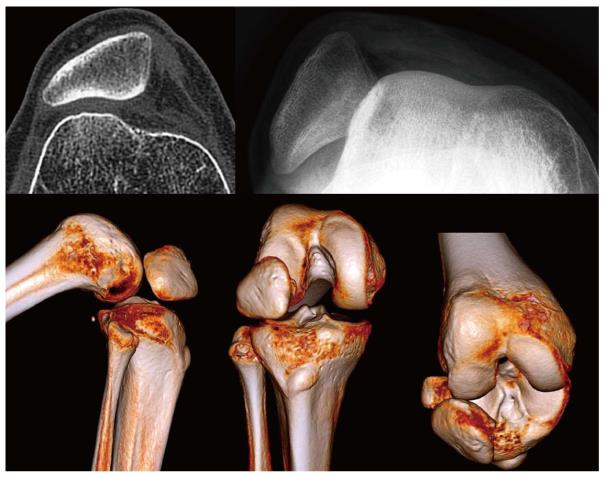
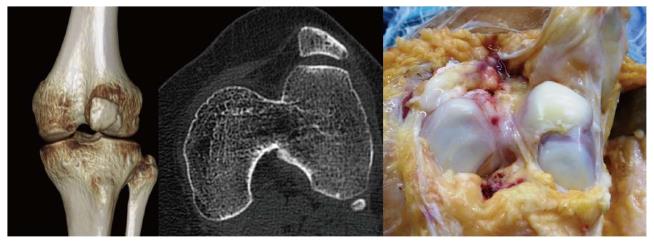
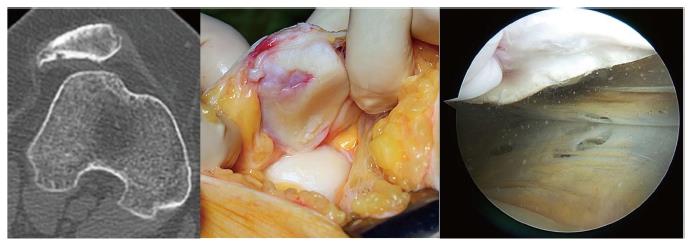
The most frequent and significant technical mistake that can lead to MPFL reconstruction failure is to position the femoral tunnel incorrectly although we can see both an incorrect femoral fixation point associated with an incorrect patellar fixation point in some cases (Figure 3). Femoral fixation point is crucial as it determines the length change behavior of the graft and therefore the graft tension at different angles of knee flexion, that is, it determines the kinematic behavior of the graft[8]. A normal MPFL is tighter in extension than in flexion. If the graft tightens when the knee is flexed, stiffness, pain and patellar overload will occur[8]. This situation typically occurs when the femoral fixation point is placed excessively anterior. In the mid-term, it may produce a severe patellar chondropathy (Figure 4) and patellofemoral osteoarthritis in the long-term (Figure 5). Therefore, it is essential to accurately check the femoral tunnel placement intra-operatively.
An incorrect femoral fixation point can lead to excessive obliquity of the graft, making it ineffective in preventing an excessive lateral patellar displacement in the first 40 degrees of knee flexion. This would explain a persistent lateral dislocation of the patella with a healthy graft. In this case, correction of the instability can be accomplished simply by modifying the fixation points despite the presence of additional anatomical factors predisposing to lateral instability such as severe trochlear dysplasia (Figure 6).
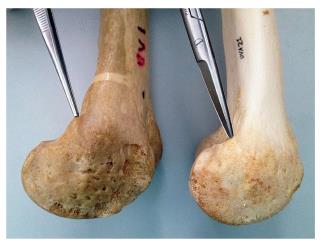
Schöttle et al[9] have recommended the used of intraoperatory fluoroscopy to more accurately placed the femoral tunnel. Obtaining a true lateral image intra-operatively is imperative when using this radiographic method. Unfortunately, this is not always easily accomplished. In addition, several authors have observed that Schoettle’s radiological method, universally accepted as the gold standard, does not guarantee a true anatomical fixation point in many cases[10] even with the use of a true lateral radiograph[11]. The radiological method is only an approximation and should not be the sole basis for femoral attachment location. The most accurate method for pinpointing anatomic placements is to perform a large enough incision to identify the most relevant anatomic landmarks. In this case, it is the adductor magnus tendon (AMT). The AMT is readily identified and leads right to the MPFL origin on the femur, situated 10.6 ± 2.5 mm distal to the apex of the adductor tubercle and parallel to the long axis of the femur[12]. The great variability in the location of the adductor tubercle (Figure 7) explains the variability in the location of the femoral insertion of the MPFL. This explains the large number of errors when using Schoettle’s method to identify the femoral anatomic fixation point of the MPFL.
Relative to the MPFL patellar insertion site, Kikuchi et al[13] have recently shown that it is largely consistent. Most of its fibers insert more into the vastus medialis obliquus (VMO) and vastus intermedius than into the patella. Unlike the femoral fixation point, accuracy in placing the patellar fixation has been shown to be less important[8]. In fact, the MPFL length changes depend on the femoral attachment site more than on the patellar attachment site[8].
Another technical error that can lead to surgical failure is excessive graft tension. The concept of ‘‘tensioning’’ the MPFL graft is not correct from a conceptual point of view given that in its native state the MPFL is not under constant tension[5]. It only comes under tension when a lateral force acts on the patella displacing it laterally. Philip Schoettle makes a very intelligent simile, comparing the MPFL to a dog leash. The leash is loose most of the time, except when the dog (the patella) wants to run away (dislocate), and then it becomes tight. If the leash (the MPFL) were tight all the time, it would choke the dog. Continuing with our simile, it would create a high patellofemoral pressure that would lead to osteoarthritis. In vivo, MPFL kinematic studies have shown that MPFL length was longest from 0° to 60° of knee flexion and decreased significantly during flexion from 60° to 120°, thereby checking excessive patellofemoral compression force during high degrees of knee flexion[8]. Additionally, the MPFL is not tight when the patella is not subject to a lateral displacing force[5].
Use the trochlea to reduce the patella when the graft is fixed by having the patella fully engaged in the trochlea at this point - 30° of knee flexion is generally sufficient to accomplish it[8]. Do not pull the graft tight at the time of fixation. If the other knee is asymptomatic, the aim is to reproduce the degree of patellar mobility of the contralateral healthy knee. We must note that tighter is never better in this operation.
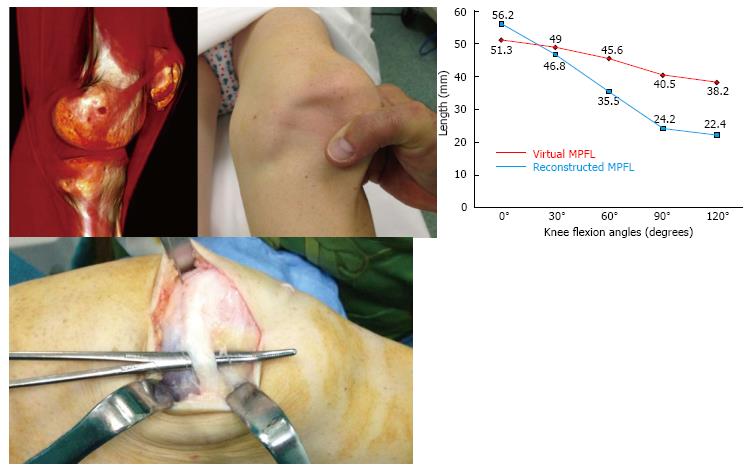
In Figure 8, you can see a failed MPFL reconstruction due to poor positioning of the femoral fixation point. The value of this particular clinical case is threefold. First, there are no confusion variables that can influence the result as the most important factors predisposing to instability were normal (no patellar tilt, no patella alta, normal TT-TG distance, and no trochlear dysplasia). Secondly, the contralateral knee was operated on with an excellent result, and therefore we were able to compare the femoral fixation point of the failed operated knee with the successfully treated contralateral knee. In the third place, the patient was a professional athlete with high demand on her knees and therefore the surgical precision had even a greater role. While minor surgical malpositioning of the femoral tunnel might be well tolerated in non-athlete patients, it is not the case in an athlete. The only differentiating factor between both knees was the position of the femoral fixation point, with maximum physical demand of both knees.
Figure 8 shows the case of a 20-year-old female, a professional classical and contemporary ballet dancer, operated on for lateral patellar instability in both knees, secondary to an obvious trauma during sport practice. She had had two clear dislocation episodes in each knee, one of which required a reduction in the emergency department. A double bundle semitendinosus reconstruction was performed in her left knee with an excellent result at 10 years after surgery. A single bundle partial thickness quadriceps tendon reconstruction was performed in the right knee. One year and a half after surgery, she complained of severe disability while practicing sports with pain and instability. She had AKP that caused her to develop defense mechanisms during physical activities to mitigate the pain. They included avoiding full knee extension while doing splits, avoiding performing full squats and squatting with the upper body flexed forward in order to reduce patellofemoral compression force and therefore the pain. She also showed a very severe patellofemoral crepitus and pain with knee flexion. She also had instability and apprehension. To perform “spiral twists” in her classical ballet activity, she avoided knee flexion from 0 to 30 degrees because of the fear that the patella “would slip laterally”. So, she also developed a defense mechanism against instability. She was then operated on again on the right knee. A semitendinosus double bundle graft was performed with an anatomic femoral fixation point. Four years after surgery, the clinical outcome was excellent. She was pain- and instability-free and was involved in high-level competitive sports with no limitations. Additionally, the previous severe painful crepitus completely disappeared.
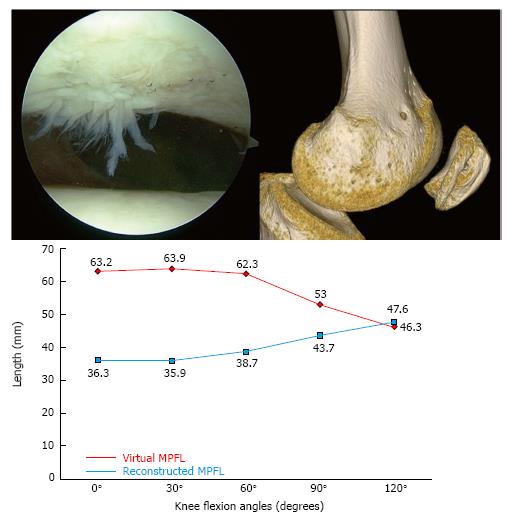
When we consider a revision surgery in a patient with a failed MPFL reconstruction, a dynamic 3D - Computed Tomography (CT) study at 0°, 30°, 60°, 90° and 120° of knee flexion to evaluate the kinematic behavior of the graft in vivo[5,8] should be performed. In the left knee of the patient in Figure 8, the length change behavior of the graft, although non-anatomic, was similar to that of a graft fixed anatomically in the femur, which is isometric from 0° to 30° of knee flexion. However, the first surgery performed on the right knee with a non-anatomic technique showed an isometric behavior between 0° to 120° of knee flexion, clearly different to the native anatomic MPFL. Therefore, a non-anatomic femoral fixation point is not necessarily associated with a failed reconstruction. In other words, if a patient with a reconstruction with a non-anatomic femoral fixation point which behaves physiologically has pain and instability, we must rule out other causes than the MPFL femoral fixation point as responsible for the pain and/or instability[8].
After performing an anatomic femoral fixation point during the revision surgery in the right knee the result was excellent, with the resolution of pain, crepitus, and instability. Therefore, we can conclude that the technical error in placing the femoral tunnel too anteriorly was the cause of the failed surgery in the right knee. On the other side, the left knee operated on with a non-anatomic femoral fixation point showed excellent outcome at ten years of follow-up. This gives rise the following question: Is the anatomic femoral tunnel position so relevant in MPFL reconstruction?
Femoral tunnel malposition does not always lead to a poor outcome[8,14]. In our experience, those ligaments with a non-anatomic femoral fixation point that behave kinematically as an anatomic MPFL, as occurs in the left knee of our “case example”, are those with an excellent clinical outcome at long-term follow-up[8,14,15]. However those non-anatomic grafts that do not have a physiologic kinematic behavior, as in the right knee, are those that have a poor clinical outcome[8]. Therefore, what should we do? We believe every MPFL graft should be placed anatomically, because an anatomic femoral tunnel position maximizes outcomes and provides the best chance of excellent short-term and long-term success. In summary, an anatomic MPFL reconstruction of the MPFL is a fast and reproducible way to achieve an MPFL that is long enough to act as an isometric “leash” from 0° to 30° and becoming loose after 30° of knee flexion. In conclusion, to avoid complications, the relevant anatomy and biomechanics must be identified and restored.
Instability occurs between 0° and 30° range of knee flexion in about 85% of the cases of CLPI. In these degrees of range of motion, patellar stability against the lateral displacing forces of the patella relies mainly on the MPFL[5]. Beyond 30° of knee flexion, the stability of the patella mainly depends on the bony anatomy of the femoral trochlea. While an isolated MPFL reconstruction it is sufficient in most cases in the former group of patients, this might fail to control the instability in the second group. Surgical failure in MPFL reconstructions are due to incorrect diagnosis where non-treatment of additional lateral patellar instability risk factors such as trochlear dysplasia are not addressed. Apprehension that is relieved at 30° of knee flexion suggests a good clinical result with an isolated MPFL reconstruction. An apprehension beyond 60° of knee flexion suggests a severe trochlear dysplasia, or a significant patella alta or both.
The surgical treatment of a patient with lateral patellar instability should be an individualized treatment as the Lyon School advocates. Awareness of the major risk factors for the development of CLPI (trochlear dysplasia, patella alta, TT-TG distance greater than 20 mm and patellar tilt greater than 20°) is required[16]. Among all these factors, the most relevant is trochlear dysplasia. Interestingly, Nelitz et al[17] observed that severe trochlear dysplasia (Dejour type B-D) was significantly more frequent in the surgical failure group (89%) than in the non-surgical failure group (21%) in an analysis of failed surgery for patellar instability. However, they did not find differences relative to the patellar height ratio (Insall-Salvati index) and the TT-TG distance between the two groups. Considering that trochlear dysplasia seems to be a major risk factor for failure of operative stabilization of CLPI, reconstruction of the MPFL as well as trochleoplasty should be considered in such cases. Wagner et al[18] also found that high degrees of trochlear dysplasia correlate with poor clinical outcome because the MPFL graft might be overloaded given that there is more instability in dysplastic situations. They conclude that trochleoplasty must be considered in cases with high degrees of trochlear dysplasia. However, this conclusion was only based on one case series study (level of evidence IV). Similarly, Kita et al[19] reported that severe trochlear dysplasia is the most important predictor of residual patellofemoral instability after isolated MPFL reconstruction. They have shown that a combination of severe trochlear dysplasia with an increased TT-TG distance was more likely to affect the outcomes of MPFL reconstruction[19]. They also suggested that additional stabilization procedures should be performed in the surgical treatment of such patients. Matsushita et al[20] demonstrated that isolated MPFL reconstructions performed in CLPI with a TT-TG distance greater than 20 yielded similar clinical outcomes to those performed with a TT-TG under 20. Moreover, there were no re-dislocations in either group. They concluded that a TT-TG distance greater than 20 mm may not be an absolute indication for medialization of the tibial tubercle.
The trochleoplasty procedure not only corrects the trochlear dysplasia, but also the increased TT-TG distance.
Dejour et al[21] have shown that the sulcus-deepening trochleoplasty is an acceptable revision option for the surgical treatment of patients with persisting patellar dislocation and high-grade trochlear dysplasia. According to Fucentese et al[22] trochleoplasty is a useful and reliable surgical technique to improve patellofemoral instability in patients with a dysplastic trochlea. However, while improved stability is predictable, pain is less predictable and may even increase following surgery. Interestingly, Schöttle et al[23] have shown that the risk for cartilage damage after trochleoplasty is low. Be that as it may, overall results are directly dependent on the type of the dysplasia, with a significantly better clinical outcome in type B and D[22]. In conclusion, severe trochlear dysplasia can be successfully treated with a trochleoplasty.
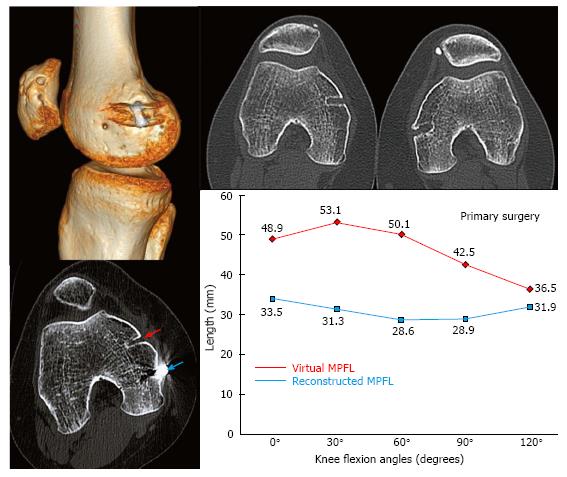
In Figure 9, a 25-year-old male patient complained of persistent instability after 2 surgical procedures for CLPI of his left knee. After the first procedure performed 2 years earlier with a single-bundle semitendinosus MPFL reconstruction, he had countless episodes of lateral patellar dislocation. In one of them, he had a patellar osteochondral fracture that was not diagnosed initially, and that brought on locking episodes. A second surgeon had recommended an arthroscopy to remove the intraarticular loose body and to perform an Insall proximal realignment surgery (overlapping of the VMO and a lateral retinaculum release). The patient did not accept this later technique. An isolated arthroscopic loose body removal and a lateral retinaculum release (LRR) were finally performed. Logically, while the locking symptoms were resolved, the instability got even worse. During physical examination, the patella could be dislocated laterally within the whole range of motion of the knee. Imaging studies showed a grade D trochlear dysplasia, a patella alta (Caton-Deschamps of 1.24), a TT-TG distance of 26 mm, and a patellar tilt of 38°. Thus, all the four major risk factors were concomitantly present.
The 3D-CT study revealed a non-anatomical femoral fixation point. However, the in vivo kinematic study of the MPFL using 3D-CT showed a graft similar in length to a native virtual ligament and an isometry from 0° to 30° similar to the native healthy ligament. We must note again that a non-anatomic MPFL reconstruction may be able to achieve an adequate change of length pattern of the graft and an optimal isometry from 0° to 30° that leads to excellent long-term clinical result[8]. Hence, the persistent pain and instability could not be attributed to this non-anatomic femoral fixation point. Thus, causes of graft failure other than the choice of the femoral fixation point should be highlighted. Type D trochlear dysplasia justified the instability at high degrees of knee flexion and might also explain the failure of the MPFL reconstruction.
Since a LRR was performed in the second surgery, medial patellar stability was also tested during the dynamic CT study. This study showed no pathological findings. Extensive LRR might lead to iatrogenic medial patellar instability or a patellar multidirectional instability that would require a reconstruction of the lateral patellar retinaculum[24-26].
Reconstruction of the deep bundle of the lateral patellar retinaculum in cases where the LRR performed in a previous surgery was too extensive should be considered.
A double bundle semitendinosus MPFL anatomic reconstruction associated with a sulcus deepening trochleoplasty was finally performed. After 4 years of follow up, the outcome was excellent.
Trochleoplasty should be only performed when the patella dislocates at high degrees of knee flexion, mostly in revision surgeries.
In this type of trochleoplasty, TT-TG distance and patellar tilt are secondarily corrected to normal physiological values. No tibial tubercle medialization or lengthening of the lateral retinaculum is needed. The remaining major instability factor, patella alta, is not addressed. However, the threshold from where the patella must be lowered remains unclear[7]. Moreover, we must note that isolated MPFL reconstruction can decrease patellar height[27]. Therefore, an isolated MPFL reconstruction may normalize patellar height in patients with CLPI and a borderline patella alta. Furthermore, we must be cautions when performing a distalization of the tibial tubercle because it always implies a certain degree of medialization (a decrease in the TT-TG distance)[28].
As to the timing of the surgical techniques, patellofemoral mal-tracking correction is needed initially. The trochleoplasty procedure fulfills the goal of neutralizing the lateral displacing forces.
Selective epidural analgesia in selected cases can help to evaluate the active patellar excursion after realignment surgery.
Once the patellofemoral joint is realigned, the second step is to stabilize the joint, which means restoring the passive restraining structures. In this second step, we perform an MPFL reconstruction.
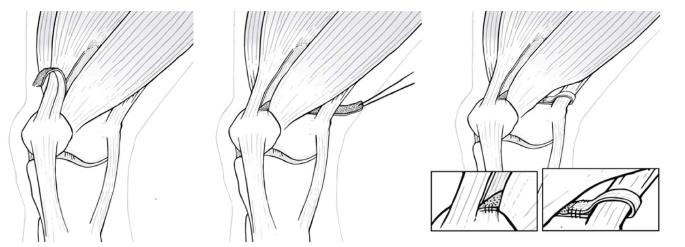
In some infrequent cases and once the MPFL has been reconstructed, patellar tilt may still show an abnormal condition. In this scenario, a third surgical step in the lateral patellar retinaculum may be necessary to achieve a good patellofemoral balance. The decision to operate or not on the lateral patellar retinaculum is an intraoperative decision, based on the patella tilt test[29].
The patella tilt test is crucial to determine the necessity for surgery on the lateral retinaculum. To do this test, a transverse K wire is placed on the proximal patella, from medial to lateral. With the knee in full extension and at 20° of flexion, the K wire should be parallel to the surgery table. If the K wire is tilted (positive test) within this range of motion, a lateral patellar retinaculum lengthening is needed. Lateral retinaculum release is only performed when lengthening is not feasibly.
Also in cases where an extensive LRR had been performed, a reconstruction of this lateral retinaculum would be necessary[26]. This surgery should only be performed after a detailed radiological assessment of medial patellar instability. Always this technique is performed after the MPFL reconstruction, since this sometimes also stabilize the patella medially (Figure 10).
To guide the patella towards the trochlear sulcus during the first degrees of knee flexion, the MPFL and the lateral retinaculum must interact in a harmonious way. Both ligaments behave similarly to a horse’s reins. The rider must hold the reins loosely, without too much tension. If not, the bit (equivalent to the patella) would press into the tongue (equivalent to the femoral trochlea), hurting the horse. However, both reins must have some degree of tension. Otherwise, it would not be possible to lead the horse to the right path.
In this section, the three anatomical factors most closely related to CLPI from an imaging point of view are analyzed.
Although lateral conventional radiography allows the evaluation of the typical signs of trochlear dysplasia[16,30], it tends to underestimate the degree of dysplasia in comparison to CT and magnetic resonance imaging (MRI)[31]. It also requires a true lateral view of the knee to avoid misinterpretation[32].
CT and MRI also provide a more accurate assessment of trochlear dysplasia. The qualitative analysis is crucial and determines the severity of the dysplasia using the classification described by D. Dejour. In addition, different quantitative measurements have been proposed to determine the depth and inclination of the trochlea in CT and MRI[33-35]. However, diagnosis of the degree of trochlear dysplasia with CT and MRI is still a challenge. Firstly, a recent study has shown that only low-grade (type A) or high-grade trochlear dysplasia (types B-D) can be reliably distinguished using Dejour’s classification, whereas the four-grade classification shows fair intraobserver and interobserver agreements[31]. Secondly, quantitative measurements of the femoral trochlea are not correlated with the Dejour’s classification of trochlear dysplasia and there are no reproducible methods for quantifying types B, C and D severe dysplasia[36]. And finally, some studies have revealed differences in the surface geometry of the cartilage and subchondral osseous contours with an exacerbated dysplasia due to the overlying cartilaginous morphology[37-39]. This highlights the importance of evaluating the femoral trochlea with MRI, which provides direct visualization of the cartilage and functional information of articular congruence.
Patellar height has classically been evaluated in standard radiography with the use of different indexes, such as the Caton-Deschamps, the Insall-Salvati, the modified Insall-Salvati and the Blackburne-Peel. However, these methods have many limitations. They have poor agreement and the patellar height classification relies heavily on the chosen ratio[40]. In addition, they refer to the position of the patella relative to the tibia and are based on bone contours and not on cartilaginous landmarks. Patellar height may be normal when measured on one index and abnormal when measured on another index.
Some authors have studied the “functional engagement” between the articular surfaces of the femur and tibia in sagittal MRI, which is more clinically relevant in patellofemoral disorders. Biedert and Albrecht introduced the patellotrochlear index[41]. Dejour described the sagittal patellofemoral engagement index in two distinctive sagittal slices, allowing measurements in patients with patellar dislocation who have different positions of the patella in the axial plane[42]. Some studies have demonstrated the absence of correlation between these functional engagement indexes and the other ratios for patella alta[42-44]. Nowadays, the evaluation of the functional engagement of the patella with MRI is recommended as a supplementary tool to the existing radiographic methods[42,44].
The TT-TG distance is the distance between the deepest aspect of the trochlear groove (TG) and the most anterior aspect of the proximal tibial tubercle (TT) in the center of the patellar tendon insertion, measured on axial CT and MRI views. They are routinely measured with the patient in the supine position, knees at 0° of flexion, feet at 15° of external rotation and the quadriceps muscle relaxed. A threshold of 20 mm is widely considered pathological.
Some factors significantly influence this measurement. The TT-TG distance is sensitive to knee rotation, small changes in femoral alignment and axial CT or MRI scan orientation[45,46]. In addition, low reproducibility of the measurement has been described, with an error of about 3-4 mm depending on the slices selected and the landmarks chosen by the radiologist[47]. Therefore, it should be interpreted with caution if the examination procedure and the measurement method have not been standardized.
The tibial tubercle-posterior cruciate ligament (TT-PCL) distance has been recently introduced as a measurement not influenced by the rotation of the knee or the shape of the trochlea (Figure 11)[45]. Similarly, the new TT-TG index allows for correlation of the distance with individual joint size, which is especially important in cases of marginal TT-TG distance[48]. These additional methods for determining the position of the tibial tubercle are currently recommended to facilitate the therapeutic approach.
High quality clinical studies are needed to determine the specific role of the TT-TG measurement in surgical decision-making for the treatment of CLPI.
A pathologic index, as an isolated number, is insufficient to consider an associated surgical technique to the MPFL reconstruction. Other factors must be considered, such as maltracking, chondropathy location (Figure 12), type of dislocation (traumatic vs atraumatic), bilaterality, activity level, and patient expectations. Much more controversies exist about osteotomy indications. According to Robert Teitge (personal communication) we may consider an osteotomy in cases with torsion greater than 20° above normal (femoral anteversion > 35° and tibial external torsion > 45°) that have failed after a MPFL reconstruction while pain, instead of instability, is the main symptom, and there is no osteoarthritis.
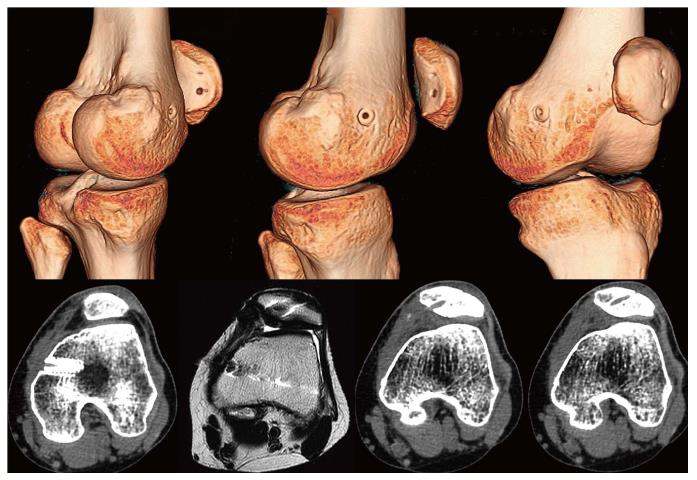
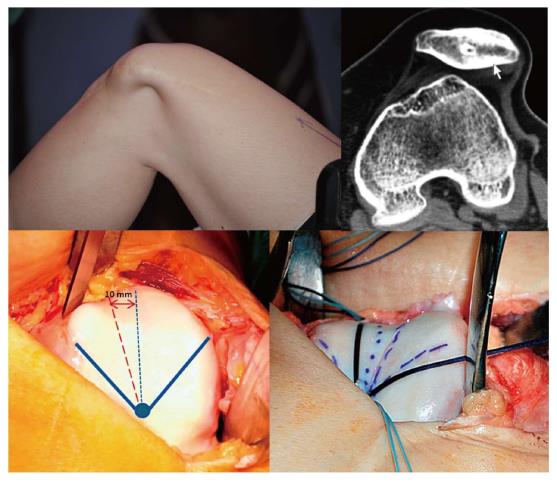
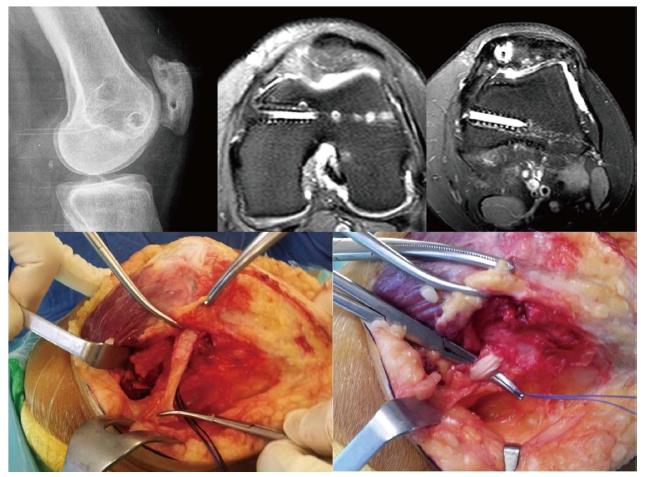
In patients who have been operated on several times, multiple tunnels and implants both in the patellar insertion area as well as in the femoral insertion area are usually seen. This situation makes revision surgery a real challenge (Figure 13), increasing the risk of patellar fractures either during or after surgery. Moreover, if we drill another tunnel in the patella or in the femur we can cause tunnel collisions that might compromise the implant fixation. This may sometimes call for a two-stage surgery as occasionally occurs in ACL revision surgery. Alternatively, we could consider a ligament reconstruction using methods that do not require anchoring bone tunnels. One option would be the use of an autologous quadriceps tendon graft which is anchored in the proximal 1/3 of the patella, maintaining its native patellar insertion site and using the AMT as a post (Figures 13 and 14). It has been reported that the AMT is a suitable point of insertion for MPFL reconstruction because the kinematic behavior exhibited by the reconstructed MPFL using either the anatomical femoral footprint of the MPFL and the AMT is similar[15]. In addition, this quasi-anatomic reconstruction using the AMT as the femoral fixation point has been shown to be safe and suitable for the treatment of CLPI and has good clinical results[14]. The advantages of this surgical technique are that there is no need to implants, no need for bone drilling and no need for allografts. These eliminate the necessity of a two-stage procedure.
The quasi-anatomical MPFL reconstruction using the adductor magnus tendon as the femoral fixation point is a good solution to deal with challenging cases in our daily practice.
The quadriceps tendon graft is harvested in its medial aspect, obtaining 1 cm width and the superficial anterior half in its complete length (Figure 14). However, the most important thing to keep in mind is to make a good estimation of the length of the graft. It should be large enough to allow for the correct isometric properties of the graft. In this regard, an extra 2 cm in the graft length to the distance between the quadriceps tendon insertion and the AMT is recommended. This will allow the graft to flip around the AMT. When the graft is harvested, dissecting the plane between the VMO and the joint capsule is a must (Figure 14). Once the graft is passed on this plane, a loop is created with its end around the AMT. Then, the attachment of the quadriceps tendon graft into the medial rim of the patella (superior third) is fixed with sutures. This prevents the graft rupturing during posterior steps and also places the graft insertion in a more anatomical position. Finally, the quadriceps tendon graft is sutured to itself in an end-to-side fashion at 30 degrees of knee flexion (Figure 14).
Complications after MPFL reconstruction can be more disabling than the primary CLPI. Some patients who have experienced more than one patellar dislocation are still highly functional and may not need surgery. Only when patients are significantly limited in their activities of daily living or with more demanding activities should surgical treatment such as MPFL reconstruction be considered. We, as a professional group, need to be extremely careful recommending this procedure to patients who must be clearly informed about the complications and secondary procedures. Even though, most failed MPFL reconstructions are a result of factors that the surgeon can control. Understanding of the anatomy and biomechanics, cautiousness with the imaging techniques while favoring clinical over radiological findings and common sense to determine the adequate surgical technique for each particular case are critical steps in minimizing potential complications.
Unfortunately, while there are several national registries collecting data on anterior cruciate ligament (ACL) reconstructions, there is a lack of such registries on MPFL reconstructions. Hopefully, the same interest will be given to the MPFL surgery in the future. These registries, along with evidence based medicine promotion, and planning higher levels of evidence studies than those available today, obviously including clinical trials, will provide tools to improve the surgical indications mostly in more the challenging cases of patellofemoral instability. Given the fact that MPFL injuries are much less frequent than ACL injuries and that there are many more factors favoring patellofemoral instability, some of them acting as confounding factors, multicentric studies should be promoted.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cartmell S, Papachristou GC, Petri M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Sanchis-Alfonso V, Montesinos-Berry E, Baydal-Bertomeu JM, Garrido-Jaen D. Are kinematic and kinetic analyses useful to evaluate patellofemoral disorders in clinical practice? J Biomed Eng Med Devic. 2016;1:105. [DOI] [Cited in This Article: ] |
| 2. | Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40:1916-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41:1030-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Schneider DK, Grawe B, Magnussen RA, Ceasar A, Parikh SN, Wall EJ, Colosimo AJ, Kaeding CC, Myer GD. Outcomes After Isolated Medial Patellofemoral Ligament Reconstruction for the Treatment of Recurrent Lateral Patellar Dislocations: A Systematic Review and Meta-analysis. Am J Sports Med. 2016;44:2993-3005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Weinberger JM, Fabricant PD, Taylor SA, Mei JY, Jones KJ. Influence of graft source and configuration on revision rate and patient-reported outcomes after MPFL reconstruction: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Sanchis-Alfonso V. How to Deal With Chronic Patellar Instability: What Does the Literature Tell Us? Sports Health. 2016;8:86-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Domenech J, Martí-Bonmatí L. Femoral insertion site of the graft used to replace the medial patellofemoral ligament influences the ligament dynamic changes during knee flexion and the clinical outcome. Knee Surg Sports Traumatol Arthrosc. 2015; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 444] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24:2838-2844. [PubMed] [Cited in This Article: ] |
| 11. | Ziegler CG, Fulkerson JP, Edgar C. Radiographic Reference Points Are Inaccurate With and Without a True Lateral Radiograph: The Importance of Anatomy in Medial Patellofemoral Ligament Reconstruction. Am J Sports Med. 2016;44:133-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Fujino K, Tajima G, Yan J, Kamei Y, Maruyama M, Takeda S, Kikuchi S, Shimamura T. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23:998-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Kikuchi S, Tajima G, Yan J, Kamei Y, Maruyama M, Sugawara A, Fujino K, Takeda S, Doita M. Morphology of insertion sites on patellar side of medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Monllau JC, Masferrer-Pino À, Ginovart G, Pérez-Prieto D, Gelber PE, Sanchis-Alfonso V. Clinical and radiological outcomes after a quasi-anatomical reconstruction of medial patellofemoral ligament with gracilis tendon autograft. Knee Surg Sports Traumatol Arthrosc. 2015;26704788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Pérez-Prieto D, Capurro B, Gelber PE, Ginovart G, Reina F, Sanchis-Alfonso V, Monllau JC. The anatomy and isometry of a quasi-anatomical reconstruction of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;26581363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1326] [Cited by in F6Publishing: 1231] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 17. | Nelitz M, Theile M, Dornacher D, Wölfle J, Reichel H, Lippacher S. Analysis of failed surgery for patellar instability in children with open growth plates. Knee Surg Sports Traumatol Arthrosc. 2012;20:822-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21:318-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Kita K, Tanaka Y, Toritsuka Y, Amano H, Uchida R, Takao R, Horibe S. Factors Affecting the Outcomes of Double-Bundle Medial Patellofemoral Ligament Reconstruction for Recurrent Patellar Dislocations Evaluated by Multivariate Analysis. Am J Sports Med. 2015;43:2988-2996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Matsushita T, Kuroda R, Oka S, Matsumoto T, Takayama K, Kurosaka M. Clinical outcomes of medial patellofemoral ligament reconstruction in patients with an increased tibial tuberosity-trochlear groove distance. Knee Surg Sports Traumatol Arthrosc. 2014;22:2438-2444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Dejour D, Byn P, Ntagiopoulos PG. The Lyon’s sulcus-deepening trochleoplasty in previous unsuccessful patellofemoral surgery. Int Orthop. 2013;37:433-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Fucentese SF, Zingg PO, Schmitt J, Pfirrmann CW, Meyer DC, Koch PP. Classification of trochlear dysplasia as predictor of clinical outcome after trochleoplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19:1655-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Schöttle PB, Schell H, Duda G, Weiler A. Cartilage viability after trochleoplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31:422-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Sanchis-Alfonso V, Merchant AC. Iatrogenic Medial Patellar Instability: An Avoidable Injury. Arthroscopy. 2015;31:1628-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Andrish J. Deep Transverse Lateral Retinaculum Reconstruction for Medial Patellar Instability. Arthrosc Tech. 2015;4:e245-e249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21:1180-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15:61-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Arendt EA. Lateral-sided surgery with MPFL reconstruction: When is this needed? Montpellier: La Patella, Sauramps Medical 2012; 119-123. [Cited in This Article: ] |
| 30. | Malghem J, Maldague B. Depth insufficiency of the proximal trochlear groove on lateral radiographs of the knee: relation to patellar dislocation. Radiology. 1989;170:507-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lippacher S, Dejour D, Elsharkawi M, Dornacher D, Ring C, Dreyhaupt J, Reichel H, Nelitz M. Observer agreement on the Dejour trochlear dysplasia classification: a comparison of true lateral radiographs and axial magnetic resonance images. Am J Sports Med. 2012;40:837-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Koëter S, Bongers EM, de Rooij J, van Kampen A. Minimal rotation aberrations cause radiographic misdiagnosis of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2006;14:713-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Biedert RM, Bachmann M. Anterior-posterior trochlear measurements of normal and dysplastic trochlea by axial magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc. 2009;17:1225-1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Pfirrmann CW, Zanetti M, Romero J, Hodler J. Femoral trochlear dysplasia: MR findings. Radiology. 2000;216:858-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 251] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Carrillon Y, Abidi H, Dejour D, Fantino O, Moyen B, Tran-Minh VA. Patellar instability: assessment on MR images by measuring the lateral trochlear inclination-initial experience. Radiology. 2000;216:582-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 224] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Nelitz M, Lippacher S, Reichel H, Dornacher D. Evaluation of trochlear dysplasia using MRI: correlation between the classification system of Dejour and objective parameters of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22:120-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Stäubli HU, Dürrenmatt U, Porcellini B, Rauschning W. Anatomy and surface geometry of the patellofemoral joint in the axial plane. J Bone Joint Surg Br. 1999;81:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Shih YF, Bull AM, Amis AA. The cartilaginous and osseous geometry of the femoral trochlear groove. Knee Surg Sports Traumatol Arthrosc. 2004;12:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | van Huyssteen AL, Hendrix MR, Barnett AJ, Wakeley CJ, Eldridge JD. Cartilage-bone mismatch in the dysplastic trochlea. An MRI study. J Bone Joint Surg Br. 2006;88:688-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Seil R, Müller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Dejour D, Ferrua P, Ntagiopoulos PG, Radier C, Hulet C, Rémy F, Chouteau J, Chotel F, Boisrenoult P, Sebilo A. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99:S391-S398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193:1361-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17:1412-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40:1119-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 46. | Yao L, Gai N, Boutin RD. Axial scan orientation and the tibial tubercle-trochlear groove distance: error analysis and correction. AJR Am J Roentgenol. 2014;202:1291-1296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Lustig S, Servien E, Aït Si Selmi T, Neyret P. [Factors affecting reliability of TT-TG measurements before and after medialization: A CT-scan study]. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:429-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Hingelbaum S, Best R, Huth J, Wagner D, Bauer G, Mauch F. The TT-TG Index: a new knee size adjusted measure method to determine the TT-TG distance. Knee Surg Sports Traumatol Arthrosc. 2014;22:2388-2395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |