IMAGING PRINCIPLES
Prenatal 2-D and 3-D ultrasound for spinal anomaly detection
While some anomalies in the spina bifida spectrum result in open neural tube defects and therefore may be initially detected with elevated maternal serum alpha fetoprotein, skin covered (closed) spinal dysraphism and other spinal anomalies such as diastematomyelia, vertebral segmentation anomalies, sacral agenesis, spinal dysgenesis, spondylothoracic or spondylocostal dysplasia, or some skeletal dysplasias may be detected only by prenatal imaging. Routine fetal sonography has not been mandated in the United States; however, most pregnant women have at least one detailed anatomical evaluation usually occurring between 18-24 wk of gestation. More recently, routine first trimester ultrasound and maternal serum screening has been offered to patients between 11 and 14 wk of gestation with the goal of measuring the fetal nuchal translucency thickness, nasal bone length and ductus venosus waveform. Imaging at this early time point in the hands of an experienced practitioner may allow for detection of fetal structural anomalies, such as neural tube defects[1]. One metric that has been used in this timeframe is biparietal diameter, which has been shown to measure less than the 5th percentile in 50% of spina bifida patients[1].
Neural element involvement in cases of spinal dysraphism may only be detectable by ultrasound with a high frequency linear transducer[2]. However, more typical obstetrical ultrasound transducers (6-8 MHz) may still detect neural tube defects or abnormal limb positioning, which can serve as an early indicator of neurological dysfunction. Three-dimensional ultrasound of the fetal spine can have great diagnostic benefit, as it allows the operator to manipulate data in any plane after the completion of the exam, particularly if fetal positioning during the optimized 2-D acquisition is challenging[2]. In many cases, however, the presence of the obligatory cranial anomalies associated with an open neural tube defect, described in further detail below, are more readily observed than the spinal defect itself. In general, ultrasonography is able to detect vertebral segmentation, laminar segmentation or rib developmental anomalies as well as gross alignment abnormalities. Congenital fusion or spinal stenosis on the other hand may be better appreciated with MRI.
Prenatal MRI of spinal anomalies
MRI can be used prenatally to better delineate involvement of neural elements associated with osseous spinal anomalies[3]. This can be important for delivery planning, decisions regarding pregnancy interruption, and in some cases, preparation for in utero surgery. Patients with closed neural tube defects have a better postnatal prognosis, including superior bladder functionality and lower risk of scoliosis, than their open neural tube defect counterparts, and MRI may more clearly distinguish these two populations[2,4]. While it is not typically utilized for screening purposes, in some cases, fetal MRI may also demonstrate unsuspected spinal anomalies in fetuses imaged for evaluation of other organ systems.
Fetal MRI provides superior soft tissue contrast. However, fetal movement can make MR imaging in standard planes challenging, and fast pulse sequences such as steady state free precession, half-fourier acquisition single-shot turbo spin echo, fast T1-weighted gradient echo, and echo planar imaging are often utilized. Thin slices of 2-4 mm thickness can minimize the detrimental effects of fetal movement on image quality while maximizing detail of the spinal and cranial anomalies[2,3]. Standard fetal MRI is performed at 1.5 or 3 Tesla field strength. Gadolinium-based contrast agents are not administered to pregnant women, given their ability to cross the placenta and the potential toxicity of unchelated gadolinium to the developing fetus[5]. MRI can be used to approximate the location of the conus, and identify a lipoma or other soft tissue mass within the spine, however can often not detect a syrinx, fatty filum or be used to assess spinal alignment.
Postnatal imaging of spinal anomalies
Additional imaging in the postnatal period can be useful in evaluating the newborn with vertebral anomalies noted on pre-natal imaging. Plain radiographs (AP and lateral of the entire spine including the ribs), should be obtained early, optimally in the first 2 mo, as the bony details of a prenatally-noted anomaly are more evident before further ossification of the vertebra, and other anomalies not seen prenatally can sometimes be detected. All congenital vertebral anomalies have an increased risk (approximately 15%) of intraspinal anomalies. Neonatal spinal ultrasound performed before extensive laminar ossification has occurred (6-12 wk) will show major intraspinal anomalies and tethering[6]. MRI of course better demonstrates intraspinal anomalies[7-9], but generally requires a general anesthetic or heavy sedation which has theoretical deleterious effects in the young child[10-12]. MR can sometimes be accomplished with a “feed and wrap” technique in the infant without general anesthesia or sedation. This technique is used to soothe the child in an attempt to obtain the imaging without requiring sedation. When and whether all vertebral anomalies should be evaluated postnatally with MRI for intraspinal anomalies is debated. Certainly any patient with clinical signs of intraspinal anomaly (extremity deformity or bladder function suggestive of neurologic abnormality) or about to have surgery of the spine should have a whole spine MR. Otherwise the MR may be deferred until an older age when easier to perform or may possibly never be needed if the anomaly is minor without neurologic symptoms. Evaluation of the neonatal spine is typically performed with ultrasound and radiography, though MRI sometimes plays a role as well. While radiographs may readily identify bony abnormalities in the case of vertebral segmentation or formation, ultrasound or MRI better assesses the relationship to neural elements.
CONGENITAL SPINAL ANOMALIES
Chiari malformation
Chiari malformations (CM) describe a heterogeneous group of conditions that are likely embryologically unrelated. Types I and II CM both involve displacement of the cerebellum with (type II) or without (type I) displacement of the lower brainstem caudal to the foramen magnum[13]. The resulting compression of these structures can affect neurologic function and impede the normal flow of cerebrospinal fluid. CM type II is thought to be due to intrinsic structural anomalies of the brain and spinal cord during fetal development in relation to an accompanying neural tube defect[14,15]. Genetic, nutritional, and environmental factors have all been demonstrated to play a role in the evolution of these defects.
Type I CM involves extension of the cerebellar tonsils into the foramen magnum and may be discovered incidentally, often later in childhood or even adulthood and often does not require further treatment. Type II CM (previously referred to as Arnold-Chiari malformation) is certainly the more commonly diagnosed prenatal condition of the two. Affected fetuses have a small posterior fossa with hindbrain malformation consisting of displacement of the lower brainstem and cerebellar tonsils and vermis through an enlarged foramen magnum, tectal beaking, medullary kink, enlarged massa intermedia, callosal dysgenesis, and towering cerebellum through an enlarged tentorial hiatus[16,17]. This is almost always found in association with a myelomeningocele. Chiari III malformation is serious though fortunately rare and may be diagnosed prenatally after identification of an occipital encephalocele containing herniated cerebellum in addition to the findings of Chiari II. Chiari IV malformation is also extremely rare and demonstrates cerebellar hypoplasia with normal positioning of the cerebellar tonsils.
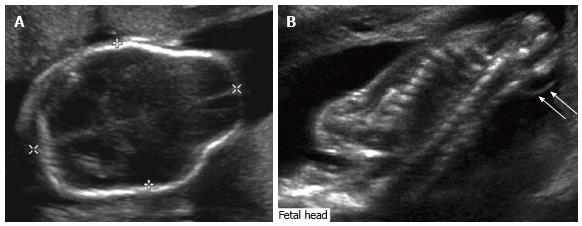
Prenatal findings: Ultrasonographic identification of CM in utero can provide valuable information regarding associated conditions. For example, ventriculomegaly is the most common prenatal finding associated with Type II CM and can be associated with neurologic dysfunction, structural abnormalities of the skull, and poor fetal outcome. Biconcave frontal bones resulting in the “lemon sign” on ultrasound (Figure 1) are often seen, though are not specific to an open neural tube defect; 1%-2% of normal fetuses may have this finding, and it may resolve over time even in patients with open neural tube defects[2]. Curved, diminutive appearance of the cerebellum due to effacement of the cisterna magna results in the typical “banana sign” on ultrasound (Figure 2), which has higher specificity for the diagnosis and is 99% sensitive. When combined, the lemon and banana signs have a > 99% sensitivity for CM type II prenatally[16]. In addition, there is an almost invariable association with myelomeningocele. While CM type II is typically diagnosed with prenatal ultrasound, fetal MRI (Figure 3) allows for confirmation, further evaluation if ultrasound is technically limited, and evaluation of associated intracranial findings such as callosal agenesis and hydrocephalus[18].
Figure 1 Fetal ultrasound.
Grayscale ultrasound images of the fetal head (A) and spine (B) demonstrate ventriculomegaly as well as a lemon-shaped configuration of the fetal head due to bifrontal concavity, typical of Chiari II malformation. There is an associated myelomeningocele (arrows).
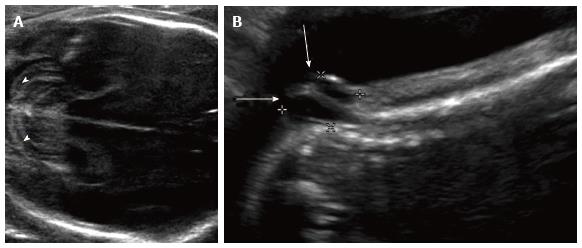
Figure 2 Chiari ultrasound.
Grayscale ultrasound images of the 23 wk fetal head (A) and spine (B) demonstrate curved, “banana-shaped” appearance of the fetal cerebellum (arrowheads) typical of Chiari II malformation, as well as associated lower sacral myelomeningocele (arrows).
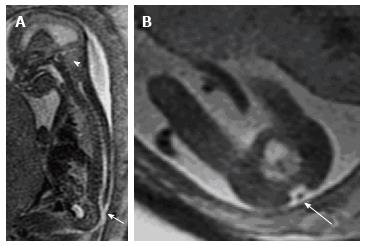
Figure 3 Fetal magnetic resonance imaging.
A: Sagittal T2 weighted fetal MR demonstrates a small posterior fossa (arrowhead) with crowding of the cerebellar tonsils and hindbrain; B: Evaluation of the spine demonstrated a lumbosacral myelomeningocele.
Postnatal evaluation and treatment: For all types of CM, findings are usually first diagnosed with MRI, which demonstrates the classic findings described above. Neurosurgical consultation is warranted in patients with CM to consider decompression of the hindbrain or treatment for hydrocephalus. Intervention for Chiari type I in the infant should generally not be entertained without a compelling clinical indication, such as lower cranial nerve dysfunction (e.g., apnea or dysphagia) or hydromyelia (syringomyelia). Such is uncommon in young infants. The classic symptoms, suboccipital and posterior neck pain exacerbated by activities involving a valsalva maneuver, cannot be appreciated in babies, and in very young children operative intervention for pain symptoms alone should be approached with caution. This is a dynamic malformation resulting from a relative deficit in the posterior fossa volume, which can change with further growth and development. In other words, for very young children, the malformation can resolve spontaneously over time. Furthermore, infants undergoing posterior fossa decompression for Chiari are more likely to have recurrence from scarring and bone regrowth, requiring repeat surgery in the future.
Patients with Chiari I malformation can present with hydromyelia (syringomyelia) even without other symptoms. Hydromyelia, or expansion of the central canal of the spinal cord (not unlike the ventriculomegaly of hydrocephalus), results from disturbed CSF flow and pulsatility at the level of the craniocervical junction in these patients. This commonly presents as progressive scoliosis, which is often “atypical” (e.g., levoscoliosis, young age, or male gender), and the diagnosis is made by MRI as part of the scoliosis workup. Chiari decompression (suboccipital craniectomy, C1 laminectomy, and duraplasty) results in resolution of the hydromyelia in most cases. If the scoliosis is not yet severe, the curve progression can be arrested or even reversed without orthopedic intervention. Untreated Chiari I with hydromyelia can lead to indolently progressive neurological deterioration involving both upper and lower motor neuronal dysfunction.
Infants with myelomeningocele and the type II Chiari malformation are quite distinct from those with Chiari I. The latter is rarely associated with hydrocephalus, while the former most commonly is. Furthermore, the type II malformation involves more than mechanical constriction of the hindbrain structures. This is a diffuse neuronal migrational anomaly affecting the entire central nervous system, with implications for neurocognitive and motor development in addition to the possibility of intrinsic brainstem dysfunction apart from the effects of mechanical compression.
The initial approach to treating symptoms of hindbrain dysfunction (e.g., stridor, dysphagia, apnea) or expanding hydromyelia in these infants is treatment of the accompanying hydrocephalus, which can now often be successfully accomplished endoscopically without shunt implantation[19,20]. Only when this has been satisfactorily accomplished should hindbrain decompression be considered, and at times recovery of lower cranial nerve functions is not realized even with that intervention.
In contrast to the Chiari I malformation, infants with Chiari II have an enlarged foramen magnum and the torcular and transverse sinuses are typically very low-lying. Hence, the need for suboccipital craniectomy is minimal and associated with higher risk, and the key part of the decompression is comprised of cervical laminectomies and an expansion duraplasty for dorsal decompression of the hindbrain seated in the cervical spinal canal.
Myelomeningocele
Myelomeningocele is a primary neural tube defect associated with failure of neural tube closure at some point along the developing spinal cord that results in a segmental spinal cord malformation with absence of the overlying dura, lamina, soft tissues and skin (Figure 4). There is typically a membrane covering the defect that traps the CSF[18]. It is one of the most common spinal anomalies, with a worldwide incidence of 1 in 1000 births[21]. A genetic predisposition to this condition has been identified in some cases, and supplemental maternal folic acid beginning prior to conception and extending through the first month of pregnancy decreases its incidence. Because of this, the incidence in a given population is variable depending on maternal access to prenatal care and nutrition in addition to genetic factors.
Figure 4 Myelomeningocele magnetic resonance - T2 weighted magnetic resonance image of a 26 wk fetus demonstrates a large thoracolumbar myelomeningocele (arrows) covered by skin.
The laminar defect, referred to as spina bifida, can also be seen in isolation or in the context of other skin covered anomalies of the spinal cord (Figure 5). These are often referred to as “spina bifida occulta” (as opposed to the “spina bifida aperta” of myelomeningocele). Spina bifida occulta lesions, such as filum lipoma, lipomyelomeningocele, and terminal myelocystocele are thought to be anomalies of “secondary neurulation” from errors in differentiation and migration of the caudal cell mass that occur after primary neurulation is complete. Thus, the general term, “spina bifida” is quite nonspecific, as is the term “dysraphism”, and can lead to confusion.
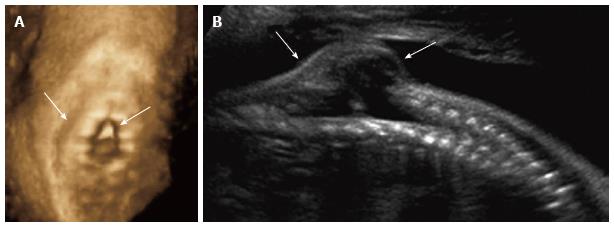
Figure 5 Spinal dysraphism ultrasound.
Coronal 3D (A) and sagittal 2D (B) ultrasound images of the 26 wk fetal spine demonstrate dysraphism of the thoracolumbar junction (arrows), in the region of known myelomeningocele.
Prenatal findings: Myelomeningocele is often first suspected when routine screening during pregnancy reveals elevated levels of maternal serum alpha-fetoprotein[22]. Targeted ultrasound also plays an important role in the early detection and delineation of neural tube defects. Myelomeningocele is virtually always accompanied by the Chiari II malformation, described above. Patients with myelomeningocele may have an additional spinal cord anomaly, such as a split cord malformation (diastematomyelia), warranting close attention to the spine in both pre- and post-natal populations for pre-surgical planning of myelomeningocele repair[16].
Prenatal treatment: While early post-natal closure of the myelomeningocele to prevent infection is the gold standard, recent advances in fetal surgery have demonstrated that prenatal open or endoscopic repair of the neural tube defect may result in reversal of the Chiari II malformation, reduced incidence of hydrocephalus, and slightly reduced severity of post-natal neurologic sequelae[23,24]. The relative value of this intervention, however, must be considered within the context of maternal and fetal risk, including the risk of prematurity and its attendant sequelae, fetal demise, and uterine dehiscence. There also appears to be an increased risk of early symptomatic spinal cord tethering and intraspinal dermoid cyst formation. In general the prevalence of orthopedic manifestations of spina bifida, including gait abnormalities, foot deformities (equinovarus, cavovarus, calcaneovalgus, vertical talus), hip dislocations, and abnormalities in the rotational alignment of the lower extremities have not been significantly impacted by prenatal interventions[21].
Postnatal evaluation and treatment: The most common early intervention aside from the initial myelomeningocele closure is the management of hydrocephalus, which is required in around two-thirds of the patients. Placement of a ventricular shunt had been the only effective treatment for these patients since the early 1960’s; but, it has recently been demonstrated that the majority (around 75%) can be successfully treated with a minimally invasive endoscopic procedure that combines endoscopic third ventriculostomy with choroid plexus cauterization[20,25].
The primary goals of orthopedic interventions should be to maximize function and mobility while minimizing deformity and pain. Treatment recommendations are based on the functional motor level of the lesion[26,27]. In patients with a thoracic or upper lumbar functional level, the goals of treatment are to maintain spinal balance, a level pelvis, mobile hips and knees, and plantigrade feet. Most of these patients will require surgical stabilization of the spine to treat scoliosis and kyphosis[28-31]. Patients with a mid-lumbar functional level usually have an intact quadriceps muscular function and have the potential to be limited community ambulators with braces and assistive devices. These patients often have weak hip abductors and develop significant genu valgum and knee flexion contractures. Muscle transfers and osteotomies to correct coronal and axial plane abnormalities play an important role in keeping these patients ambulatory[28,32]. Patients with low lumbar or high sacral function typically ambulate using ankle foot orthoses. Hip instability in this group should be aggressively managed to achieve concentric hip reduction and coverage[33]. Foot reconstruction procedures to correct cavovarus deformity are indicated to maintain a supple foot that can be braced[34,35].
Tethered spinal cord
Tethered spinal cord syndrome is a clinical entity in which spinal cord function is compromised by inappropriate attachment of and traction upon the cord, which appears to be associated with compromised tissue perfusion. Tethering of the cord is associated with a variety of etiologies, including scar formation from prior surgery (such as myelomeningocele repair) or an anomaly of secondary neurulation resulting in inappropriate conus traction from a filum lipoma or lipomyelomeningocele[36,37]. Symptoms of spinal cord tethering may also result from a split cord malformation (diastematomyelia) or from a dermal sinus tract that extends intra-dural and is attached to the dorsal spinal cord.
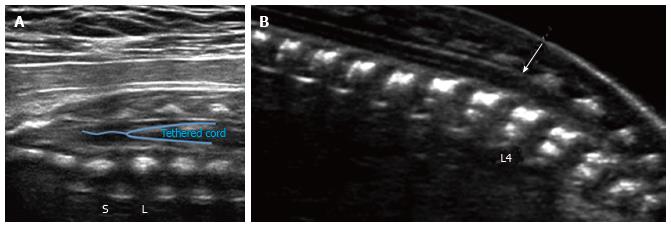
Prenatal findings:In utero diagnosis of a tethered spinal cord is usually based on direct visualization of an abnormally caudal position of the conus medullaris, or by identification of a lipoma within the spinal canal. A low-lying conus terminates below the upper endplate of L3 in a child. Though normative values in the fetus have yet to be fully established, prenatal ultrasound has demonstrated that the conus has normally ascended to the L1/L2 level by the 40th postmenstrual week (Figure 6)[38]. A tethering lesion may also result in lack of normal nerve root motion with the CSF pulsations during neonatal imaging. The diagnosis of tethered spinal cord can occasionally be made prenatally with ultrasound and fetal MRI, though postnatal diagnosis is far more common, and may be heralded by the presence of a sacral dimple or soft tissue mass, or after evaluation for symptoms related to cord tethering, such as bowel and bladder dysfunction, lower extremity orthopedic deformity, or ambulatory disturbance[17].
Figure 6 Spinal tether ultrasound.
Grayscale ultrasound image (A) of the lumbosacral spine in a 29 wk fetus demonstrates a low-lying conus medullaris, extending below the lumbosacral junction (L/S). No evidence of associated fatty filum terminale was seen in this case; B: Grayscale ultrasound image of the lumbosacral spine in a 21 wk fetus demonstrates a low-lying conus medullaris (arrow), extending below the inferior endplate of L3. No evidence of associated fatty filum terminale was seen in this case.
Postnatal evaluation and treatment: Strictly speaking, “tethered spinal cord” is a clinical rather than radiological diagnosis, and the role of a radiologist is typically to identify possible reasons for clinical symptoms of tethering. Ultrasound can be used to identify tethering up to approximately 6 mo of age, though earlier imaging is technically easier when performed before the posterior spinal elements have ossified. If a spinal tether is not suspected until the child is older, MRI is required for diagnosis.
The clinical manifestations of tethering lesions due to abnormal traction on the conus vary[39]. These include pain, neurogenic bladder dysfunction, progressive foot deformities, scoliosis, and the development of motor or sensory deficits in the lower extremities. It may be identified (as is also the case with hydromyelia) in cases where an MRI is ordered for a rapidly progressive or atypical scoliosis. The characteristic physical exam findings may include the presence of a hairy patch (associated with split cord malformations), sacral dimple, or subcutaneous lipoma in the lumbosacral region[40].
Tethered spinal cord is often released surgically as a prophylactic measure in very young children because of the anticipated risk of progressive neurologic sequelae over time. In older children, symptomatic tethering of the spinal cord may occasionally be present even if the conus is normally positioned[39], and a coexisting filum lipoma is often seen[41]. Clinical indications for tether release include back or leg pain, progressive deterioration of motor or sensory function (such as a change in gait), neurogenic bladder dysfunction, constipation, progressive orthopedic deformities, or spasticity. Rarely, patients with dermal sinus tracts may develop recurrent meningitis[42]. Although residual deficits in urologic or neurologic function may persist, these patients can have an excellent quality of life. Patients with the recent onset of clinical manifestations such as pain, constipation, or urinary incontinence often experience resolution of their symptoms.
Diastematomyelia
Diastematomyelia is a rare congenital anomaly that results in a longitudinal split of the spinal cord[43], usually occurring at the level of the upper lumbar vertebrae. The genesis of this anomaly is thought to occur very early in gestation during gastrulation and prior to neural tube closure. The two hemicords are typically separated by a fibrous, cartilaginous, or osseous septum and reside in two separate dural tubes (type I split cord malformation) (Figure 7). Type II split cord malformations have both hemicords within a single, non-duplicated, dural tube[44]. Each hemicord usually contains a central canal, one dorsal horn and one ventral horn. The two hemicords typically reunite caudally, though two coni medullarae may be seen in diplomyelia, an embryologically distinct entity[45]. Diastematomyelia is typically associated with vertebral segmental anomalies.
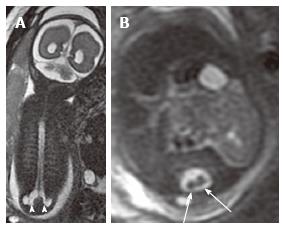
Figure 7 Lumbosacral myelomeningocele.
Coronal (A) and axial (B) T2 weighted magnetic resonance images of a 26 wk fetus demonstrate complex spinal anomalies with a lumbosacral myelomeningocele (arrowheads) and diastematomyelia (arrows).
Prenatal findings: Though the diagnosis may go unrecognized by prenatal ultrasound in some cases, the presence of a septum between the two hemicords, which appears as an echogenic structure extending from the posterior elements to the posterior aspect of a vertebral body, has high specificity for diastematomyelia[17,18,43]. The spinal canal may also be widened in the coronal plane[41]. The mean gestational age at diagnosis is 21.5 wk, and approximately one third of patients have associated spinal dysraphism[43]. Patients with isolated diastematomyelia have a far better postnatal prognosis than those with associated open neural tube defect, so identifying any of these findings prenatally is critical for family counseling. Prenatal MRI can be used to confirm the diagnosis and look for associated findings, as some patients have associated meningocele or myelomeningocele, and 75% have tethering lesions[17].
Postnatal evaluation and treatment: The clinical manifestations of diastematomyelia are very similar to those of a tethered cord. Cutaneous lesions, especially a patch of hypertrichosis, are often found over the affected area of the spine and the neurologic symptoms include motor or sensory deficits, scoliosis, incontinence and pain. Postnatal neurologic deficits may present as the child learns to walk, at which time evaluation with MRI is appropriate. MRI is used to confirm or make the diagnosis. In some cases, it may be found during screening MRI for early onset or atypical scoliosis.
If an associated myelomeningocele or secondary tethering lesion such as filum lipoma is not present, the role of prophylactic operative intervention is debated, but is certainly indicated for new or progressive symptoms. When indicated, surgical intervention includes decompression of the neural elements with excision of the interposing tissue and reconstruction of the duplicated dural sacs.
Vertebral formation/segmentation anomalies
Vertebrae typically develop between the sixth to eighth weeks of gestation when two lateral chondrification centers unite to form the primary ossification center of the vertebral body[46]. A disruption in this process can lead to a failure of vertebral formation or segmentation (Figure 8). Although the exact etiology for this phenomenon is unknown, some attribute vertebral formation or segmentation anomalies to disruption of the vascular supply to that portion of the vertebral column[47]. Vertebral anomalies may occur in multiple areas within the spine and are often associated with anomalies in the extremities and other organ systems, including cardiac, genitourinary, gastrointestinal, and pulmonary[48]. They are broadly classified into categories including failure of formation or failure of segmentation.
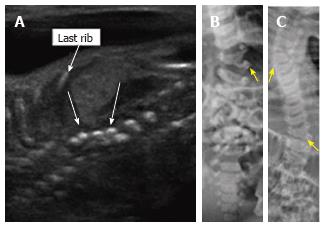
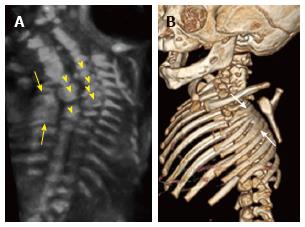
Figure 8 Thoracic hemivertebrae.
Parasagittal grayscale ultrasound image (A) of a 20 wk fetus acquired with a high frequency linear transducer demonstrate unpaired echogenic structures at the thoracolumbar junction representing hemivertebrae (arrows). Postnatal frontal radiographs of the same patient’s spine at 0 d (B) and 3 years of age (C) demonstrate left thoracolumbar and right midthoracic hemivertebrae.
Prenatal findings: Ultrasound can be used to detect a vertebral anomaly as early as the twelfth week of gestation[49]. In particular, 3-D ultrasound can have a dramatic effect on ease of visualization of these lesions, particularly in skeletal rendering mode[2]. Failures of formation, including hemivertebrae (Figure 9) and wedge vertebrae, may be identified with prenatal ultrasound or less commonly by MRI by recognizing abnormal spinal curvature and unpaired vertebral ossification centers. Failures of segmentation, including block vertebrae, unilateral bar vertebrae, and atlanto-occipital fusion are primarily related to failure of intervertebral disc formation and may present prenatally with fetal kyphoscloliosis[50].
Figure 9 Cervicothoracic Hemivertebrae.
Grayscale ultrasound image (A) of a 21 wk fetus demonstrate multiple hemivertebrae (arrows) and cervicothoracic kyphosis. Six months postnatal multidetector unenhanced computed tomography of the thorax with volume rendering (B) as well as frontal radiograph of the spine (C) demonstrate multiple vertebral segmentation anomalies, including right upper thoracic and left lumbosacral hemivertebrae (arrows), resulting in congenital cervicothoracic dextroscoliosis.
If a spine anomaly is identified, the lower extremities should be evaluated in detail for positioning and functionality. Identification of vertebral anomalies also warrants close inspection of the rest of the fetus, particularly to evaluate for VACTERL association [vertebral anomalies, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies/rib fusions (Figure 10), limb anomalies][16]. Amniocentesis can also provide valuable information regarding any chromosomal abnormalities that may be present.
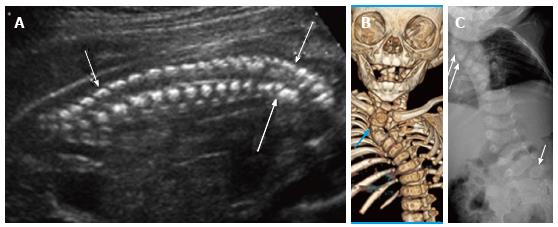
Figure 10 Rib fusions.
Grayscale 3D ultrasound image (A) of a 21 wk fetus demonstrate fusion of multiple left sided ribs (arrows) as well as multiple segmentation anomalies (arrowheads) resulting in congenital cervicothoracic kyphoscoliosis. Postnatal multidetector unenhanced computed tomography (B) of the thorax with volume rendering demonstrates bony fusion of multiple left sided ribs (arrows) as well as multiple segmentation anomalies and cervicothoracic dextroscoliosis.
Postnatal evaluation and treatment: Counseling and management of these patients depends on the gestational age at diagnosis, location and number of vertebral anomalies, and whether there is involvement of multiple organ systems. An isolated hemivertebra typically has a very good prognosis with conservative management and close follow-up. On the other hand, neonates with multiple vertebral anomalies as well as other organ system involvement are typically at higher risk for premature delivery and perinatal morbidity and mortality. Postnatal screening for associated intraspinal, cardiopulmonary, or urologic abnormalities is critical. In patients less than 3 mo of age, a spinal ultrasound may be used to identify a low-lying conus, diplomyelia, diastematomyelia or syringomyelia[51]. Intraspinal anomalies may be detected in up to 37% of patients with congenital scoliosis[52].
Congenital curves are typically inflexible and unresponsive to bracing[53]. Surgical management is indicated for documented deformity progression, and for anomalies at high risk for progression. Published data have given some insight into the relative progression of commonly encountered malformations[54]; however many of the deformities do not follow the simplified classification scheme, and serial radiographs are required during growth to understand and document progression. Additionally, the location of the vertebral anomaly also plays an important role in determining treatment. For example, small deformities at the lumbosacral or cervicothoracic junction can cause marked imbalance and large compensatory curves. Additionally the treatment of thoracic insufficiency syndrome, often seen in patients with multiple rib fusions or in patients with spondylocostal dysplasia, has recently been described as a surgical indication in patients with congenital vertebral abnormalities to prevent cardiopulmonary comorbidities and allow for symmetric development of the chest and spine[55,56].
Lumbosacral agenesis
Lumbosacral agenesis or caudal regression syndrome is a rare condition with an incidence of 1 in 25000 births. It is technically an anomaly of the caudal cell mass/filum terminale and is strongly associated with maternal hyperglycemia. Most commonly, this is seen in fetuses of mothers with preexisting uncontrolled diabetes (Figure 11) and is associated with vertebral, limb, genitourinary, and anal anomalies[39].
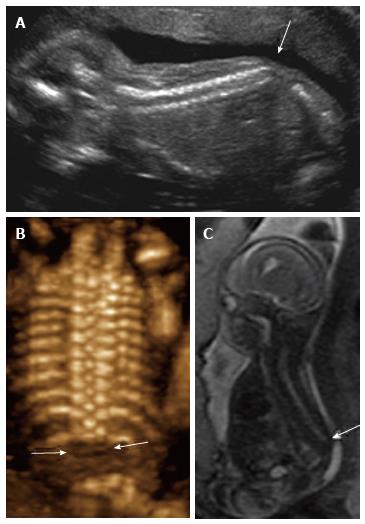
Figure 11 Sacral agenesis.
2D sagittal grayscale ultrasound (A), coronal 3D ultrasound (B), and parasagittal T1 weighted image (C) of a 19 wk fetus demonstrate absence of the fetal spine caudal to T12 (arrows), consistent with sacral agenesis/caudal regression syndrome. The mother was known to have type 1 diabetes, a risk factor for this condition.
Prenatal findings: Structurally, these patients have varying degrees of coccygeal, sacral, and lumbar agenesis with the abnormality beginning caudally and progressing cranially. The conus medullaris may have a truncated appearance in these patients both by MRI and ultrasound, and the diagnosis is usually first suspected when screening ultrasound demonstrates sacral agenesis. Recent literature suggests that conus morphology can also be evaluated in these patients prenatally with ultrasound utilizing high frequency linear transducers. Evaluation for Currarino triad is warranted in all patients with lumbosacral agenesis, which in addition to sacral anomalies, is comprised of anal atresia and a presacral mass (either teratoma or meningocele)[16].
Postnatal evaluation and treatment: The clinical manifestations of sacral agenesis can vary depending on the extent of lumbosacral root involvement[57]. Most patients have neuropathic bladder and are at increased risk for recurrent urinary tract infections and incontinence. Poor gastrointestinal motility and imperforate anus are also commonly seen. Neurologic involvement includes lower extremity muscle weakness and altered sensation[58]. Equinovarus, lower extremity contractures, kyphoscoliosis and hypoplastic pelvis with hip instability are common orthopedic manifestations of this condition.