Published online Dec 18, 2016. doi: 10.5312/wjo.v7.i12.843
Peer-review started: May 12, 2016
First decision: June 4, 2016
Revised: August 27, 2016
Accepted: September 21, 2016
Article in press: September 23, 2016
Published online: December 18, 2016
Processing time: 211 Days and 14.9 Hours
Glomus tumors are uncommon, benign, small neurovascular neoplasms derived from glomus bodies in the reticular dermis. Glomus bodies are found throughout the body to regulate body temperature and skin circulation; however, they are concentrated in the fingers and the sole of the foot. The typical presentation is a solitary nodule in the subungual or periungual area of the distal phalanx. The primary treatment of choice is surgical removal. We investigated expression of vascular endothelial growth factor (VEGF) using immunohistochemistry in glomus tumors of the fingers. All five glomus tumor samples were positive for VEGF expression. VEGF immunoreactivity was largely localized to the cytoplasm of tumor cells, suggesting a contribution of VEGF to the vascularization of glomus tumors.
Core tip: Glomus tumors are uncommon, benign, small neurovascular neoplasms derived from glomus bodies in the reticular dermis. The role of vascular endothelial growth factor has never been investigated in glomus tumors of the fingers. This case report demonstrated high vascular endothelial growth factor (VEGF) expression in the glomus tumors of the fingers, suggesting a contribution of VEGF to the vascularization of glomus tumors.
- Citation: Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop 2016; 7(12): 843-846
- URL: https://www.wjgnet.com/2218-5836/full/v7/i12/843.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i12.843
Glomus tumor is a relatively rare benign perivascular painful neoplasm arising from the neuromyoarterial structure called a glomus body, a specialized arteriovenous anastomosis involved in thermoregulation[1]. The diagnosis is virtually relied upon the basis of the clinical history and examination[2]. The classic clinical triad includes paroxysmal shooting pain, localized tenderness, and hypersensitivity to cold. Patients usually present with a painful, exquisitely tender mass beneath the nail that is accompanied by a faint bluish discoloration. Except for the slight change in color of the nail overlying the tumor, the nail may appear normal. The differential diagnosis includes local infection, osteomyelitis, osteoid osteoma, inclusion cyst, and malignancy.
Vascular endothelial growth factor (VEGF) is a crucial stimulator of blood vessel growth and is one of the most potent growth factors of angiogenesis. Angiogenesis is a fundamental process for growth of new blood vessels from preexisting vasculature during fetal development and tissue repair; however, uncontrolled angiogenesis can contribute to a variety of disorders including neoplastic diseases. In the present study, we performed immunohistochemistry to evaluate the expression of VEGF in glomus tumor tissues.
Five patients with solitary glomus tumors of the fingers were surgically treated between 2010 and 2014 at the Department of Orthopaedics of King Chulalongkorn Memorial Hospital, Bangkok, Thailand. The histological diagnoses of each tumor were validated by an experienced pathologist.
The paraffin-embedded tissues were cut in 5 μm thickness and processed for VEGF staining. Sections were deparaffinized and rehydrated in Tris-buffered saline. Endogenous peroxidase activity was blocked with 0.3% H2O2 for 10 min. For antigen retrieval, tissue sections were microwave heated in 10 mmol/L citrate buffer for 5 min. Nonspecific binding was blocked for 20 min with 3% normal horse serum (DAKO, Glostrup, Denmark), followed by incubation with primary antibody (rabbit polyclonal anti-human VEGF antibody 1:100; Santa Cruz Biotech, Santa Cruz, United States) in Tris-buffered saline containing 2% rabbit serum and 1% bovine serum albumin for two hours. Tissues were incubated with the same buffer without the antibody to serve as negative controls. Sections were subsequently stained with biotinylated goat anti-rabbit immunoglobulins (1:400; DAKO) and streptavidin/horseradish peroxidase complex (1:400; DAKO) and incubated at room temperature for 30 min. Reaction products were visualized using diaminobenzidine (Sigma, St. Louis, United States) as the chromogen. The sections were subsequently counterstained with Mayer’s hematoxylin and mounted onto microscope slides using a permanent medium.
All five glomus tumor specimens derived from five female patients (age range, thirty-five to forty-eight years) and were located on the fingers. The entire tumors were completely excised surgically.
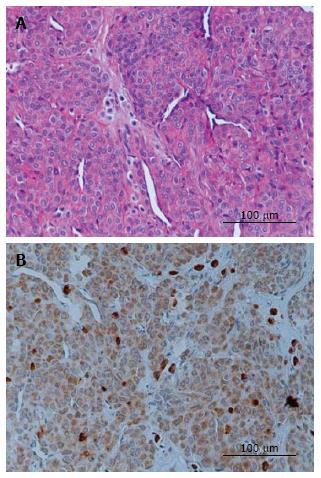
Representative sections evaluated for histological and VEGF immunostaining are demonstrated in Figure 1, and the grading is present in Table 1. Histopathologic examination demonstrated a well-defined mass to be a glomus tumor, which comprised uniform round or slightly polygonal cells with sharp cellular borders and eosinophilic cytoplasm. The cells are embedded in myxoid and collagenous stroma as solid sheets or thin layer around vascular spaces (Figure 1A). Positive VEGF staining was found in all specimens, with strong VEGF cytoplasmic staining in all five specimens (Figure 1B).
| Case | Age (years) | Clinical presentation | Finger | VEGF staining |
| 1 | 35 | Severe pain, point tenderness and bluish discoloration in subungual region | Left thumb | 3+ |
| 2 | 38 | Episodic pain, cold sensitivity and severe tenderness at the tip of digit | Left middle | 2+ |
| 3 | 40 | Excruciating pain, numbness and extreme tenderness at the tip of digit | Left small | 4+ |
| 4 | 45 | Severe pian, worse at night and bluish lesion in subungual base | Right index | 2+ |
| 5 | 48 | Paroxysmal pain, tingling, paresthesia and point tenderness at the tip of digit | Right ring | 3+ |
Immunohistochemical examination revealed that the tumor cells are strongly positive for vascular endothelial growth factor. VEGF immunoreactivity was largely localized to the cytoplasm of tumor cells (Figure 1B), suggesting a contribution of VEGF to the vascularization of glomus tumors. The patients’ symptoms including severe pain, focal tenderness, and cold hypersensitivity greatly improved and resolved entirely within several weeks following surgical removal. At present, all patients have no pain at rest or at night two years after surgery.
Glomus tumors are uncommon, small, painful, and commonly benign hamartomas arising from the arterial end of the glomus body in the reticular dermis. The typical presentation is a solitary nodule in the subungual or periungual area of the distal phalanx. Patients typically present with a triad of symptoms including excruciating paroxysmal pain (worse at night), temperature sensitivity, and severe point tenderness. Direct pressure on the tumor with the head of a straight pin or the tip of a pencil leads to excruciating pain, while pressure applied slightly to one side of it elicits no pain. Immersing the involved hand or digit in cold water or ice cube also result in discomfort.
Glomus tumors usually are less than 1 cm in diameter, often being only a few millimeters in diameter, and may be visible through the overlying tissues as a blue or purple discoloration. They occur more often in the hand nail in 25%-65% of patients. Seventy-five percent are subungual, however, these may pose a difficult diagnosing these tumors[3]. Although frequently found in the dermis, glomus tumors may occur in deep soft tissue or visceral sites throughout the body including lung, gastrointestinal, and liver[4,5]. Hypertrophy of a glomus body, an innervated, coiled, arteriovenous dermal shunt that normally controls skin temperature, is evident this tumor. Glomus cells are specialized perivascular muscle cells that are round or oval and have a dense, granular cytoplasm. Nonmyelinated nerve fibers, which are intermixed with thick-walled capillaries, are responsible for the lancinating pain. The mechanism of pain in glomus tumors has not been clearly elucidated, but it may be associated with contraction of myofilaments in response to temperature changes, resulting in an increase in intracapsular pressure[6]. Although the precise pathogenesis of glomus tumors is unknown, it is postulated that they either represent harmatomas or reactive hypertrophy secondary to trauma[6].
Glomangioma and glomangiomyoma are classic variants of the common feature of glomus tumors. The typical histological appearances of the glomus tumors comprise angiocentric uniform sheets of cells with oval nuclei, forming a perivascular collar around vessels. The three different tumor variants are differentiated by their histological characteristics. The common or solid form includes lobules, strands, and broad sheets of rounded, uniform glomus cells with indistinct capillaries in the walls of surrounding large blood vessels. Whereas glomangiomas exhibit prominent vascular structures with dilated veins surrounded by clusters of glomus cells, and glomangiomyomas consisted of prominent vascular and elongated, spindled smooth muscle cells[7]. In malignant forms, glomangiosarcoma, pleomorphic tumor cells with marked nuclear atypia and frequent mitotic figures are found in variable numbers[8]. Angiogenesis has been implicated in the progression from benign to malignant tumors. The contribution of angiogenesis and VEGF expression in glomus tumors as yet has not been completely elucidated. It is not yet clear whether there is any difference in VEGF expression between benign and malignant forms of glomus tumors.
In this study, VEGF was detectable in all specimens of glomus tumors. VEGF has been known as a potent angiogenic factor involved in neovascularization. It has been shown that VEGF are expressed in paragangliomas and may contribute to the extreme vascularity of these tumors[9]. Hence, the elevated VEGF expression in the glomus tumor might play a paracrine role in the angiogenesis that vascularizes around the tumors by capillary sprouting from the adjacent vascular network. Angiogenesis may result in the reconstruction of nutrition for the expanding tumor and could enable further proliferation. However, prospective longitudinal studies with larger sample size are warranted to define the precise role of VEGF in glomus tumors.
These tumors can be removed with the patient under local anesthesia and should be accurately localized by marking the lesion just before operation. Removal of the portion of the nail plate over the area of tenderness and excision of the matrix that appears involved along with a margin of normal-appearing matrix is the treatment of choice. Magnification and high-intensity lighting facilitate excision of these periungual and subungual masses. Meticulous and complete excision of the usually well-encapsulated lesions is curative, although rates of recurrence 4%-15% have been reported[10]. Recurrence is attributed to incomplete excision, undetected multiple tumors, or development of a new tumor.
In conclusion, increased VEGF expression was observed in glomus tumors. VEGF could contribute to the process of promoting tumor angiogenesis and might be important in the pathogenesis of glomus tumors.
Five female patients (age range, thirty-five to forty-eight years) presented with exquisite pain, point tenderness, cold hypersensitivity on their fingers.
Severe paroxysmal pain, temperature sensitivity, and severe point tenderness in the finger tips, particularly in the subungual region.
Local infection, osteomyelitis, osteoid osteoma, inclusion cyst, glomus tumor, glomangioma, glomangiomyoma, and malignancy.
All labs were within normal limits.
Glomus tumor.
Complete surgical excision of lesion.
Glomus tumor is an uncommon benign perivascular painful neoplasm arising from the neuromyoarterial structure of the glomus body. Although frequently found in the dermis, glomus tumors may occur in deep soft tissue or visceral sites throughout the body.
Glomangioma and glomangiomyoma are classic variants of the common feature of glomus tumors. Glomangiomas exhibit prominent vascular structures with dilated veins surrounded by clusters of glomus cells, and glomangiomyomas consisted of prominent vascular and elongated, spindled smooth muscle cells.
The entity of glomus tumor should not be confused with hemangioma or paraganglioma. When making a differential diagnosis of unexplained paroxysmal shooting pain, sensitivity to cold, and localized tenderness in the finger, glomus tumour must be taken into consideration. The standard treatment of choice is surgical removal. Recurrence of glomus tumor generally resulted from incomplete excision.
This is a report of vascular endothelial growth factor in glomus tumors of the fingers. The topic of this manuscript is interesting and it deserves serious consideration for publication. The high VEGF expression in the glomus tumors of the fingers has not been reported in other literatures.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ghigna MR, Munoz C, Wang PJ S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Cigna E, Carlesimo B, Bistoni G, Conte F, Palumbo F, Scuderi N. The value of clinical diagnosis of digital glomus tumors. Acta Chir Plast. 2008;50:55-58. [PubMed] |
| 3. | Lee IJ, Park DH, Park MC, Pae NS. Subungual glomus tumours of the hand: diagnosis and outcome of the transungual approach. J Hand Surg Eur Vol. 2009;34:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Ghigna MR, Fadel É, Bellini R, Rohnean A, Palazzo L, Dorfmuller P, Dartevelle P, Thomas de Montpréville V. A quite exceptional cause of recurrent hemoptysis. Chest. 2013;144:1724-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Kihara A, Fukushima J, Horiuchi H. Glomus tumor of the liver presenting as a cystic lesion. Pathol Int. 2014;64:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Samaniego E, Crespo A, Sanz A. [Key diagnostic features and treatment of subungual glomus tumor]. Actas Dermosifiliogr. 2009;100:875-882. [PubMed] |
| 7. | Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Park JH, Oh SH, Yang MH, Kim NI. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833. [PubMed] |
| 9. | Jyung RW, LeClair EE, Bernat RA, Kang TS, Ung F, McKenna MJ, Tuan RS. Expression of angiogenic growth factors in paragangliomas. Laryngoscope. 2000;110:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Gandhi J, Yang SS, Hurd J. The anatomic location of digital glomus tumor recurrences. J Hand Surg Am. 2010;35:986-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |