INTRODUCTION
Mitochondria produce over 95% of ATP through the process of oxidative phosphorylation. Under physiological conditions, mitochondria undergo a dynamic process of biogenesis, remodeling and degradation. Dysregulation of this balance results in decreased energy production, increased reactive oxygen species (ROS) and in some cases cell death. Mitochondrial dysfunction is observed with normal aging, as well as in many disease states. It is well established that damage to the central nervous system (CNS) by traumatic brain injury, spinal cord injury (SCI) and neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis) is associated with mitochondrial dysfunction[1]. Recent studies have suggested that metabolic disorders including atherosclerosis, hypertension, cancer, insulin resistance, type II diabetes and obesity is associated with decreased mitochondrial function as well[2-4]. A better understanding of the mechanisms involved in mitochondrial dynamics and ways to improve mitochondrial health could be important for designing and testing effective treatments for these clinical populations. In this editorial we will discuss the importance of studying skeletal muscle mitochondrial health and function in persons with chronic SCI.
SCI is a devastating medical condition that results from direct or indirect damage to the spinal cord. This damage can be caused by trauma or by several pathological conditions. There are approximately 12000 new cases of SCI each year in the United States and nearly half of these individuals are between the ages of 16 and 30[5]. Because of the near-normal life expectancy of persons with SCI, the estimated lifetime cost of health care and living expenses for a person with a cervical SCI is over $3 million, not including lost income[5]. After SCI, patients undergo severe body composition deterioration and skeletal muscle changes that predispose them to metabolic disorders like type II diabetes and cardiovascular disease[6-8].
MITOCHONDRIAL DYNAMICS AND ENERGY PRODUCTION
Cellular energy production
Mitochondria are double membraned organelles. The outer mitochondrial membrane (OMM) allows the passage of small molecules through voltage-dependent anion channels; however, access to the inner mitochondrial membrane (IMM) is much more tightly regulated[9]. The electron transport chain (ETC) consists of IMM-bound protein complexes I-IV and functions to maintain the electrochemical gradient across the IMM that is necessary to make ATP[10] (Figures 1 and 2). This electrochemical gradient is achieved by the pumping of protons (H+) from the mitochondrial matrix into the intermembrane space (IMS) by complexes I, III, and IV (Figure 2). Disruption of this gradient results in decreased ATP synthesis and causes electrons to leak from the ETC and react with molecular oxygen in the matrix to create the ROS superoxide (O2-)[11].
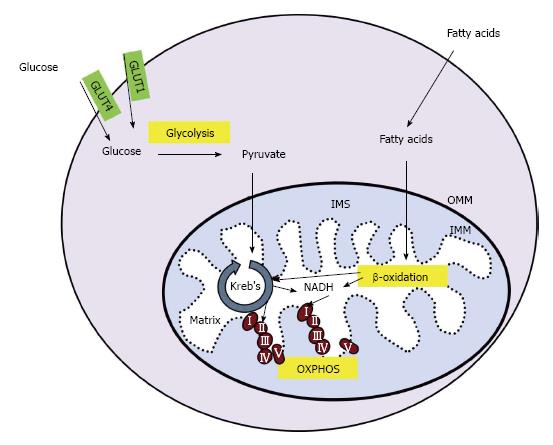
Figure 1 Cellular energy production.
In skeletal muscle, glucose enters the cell through glucose transporter type 1 or 4 (GLUT1 or GLUT4, respectively). Glucose is converted to pyruvate in the glycolysis pathway. Pyruvate is transported across the outer and inner mitochondrial membranes (OMM and IMM, respectively) and into the mitochondrial matrix where it is converted into acetyl-coA. Fatty acids undergo β-oxidation in the mitochondria, creating acetyl-coA and NADH. Acetyl-coA is utilized by the Kreb’s cycle, creating NADH and succinate which enter the electron transport chain (ETC) at complex I and II, respectively. The movement of electrons through the ETC is coupled to the production of ATP in a process called OXPHOS. IMS: Intermembrane space; OXPHOS: Oxidative phosphorylation.
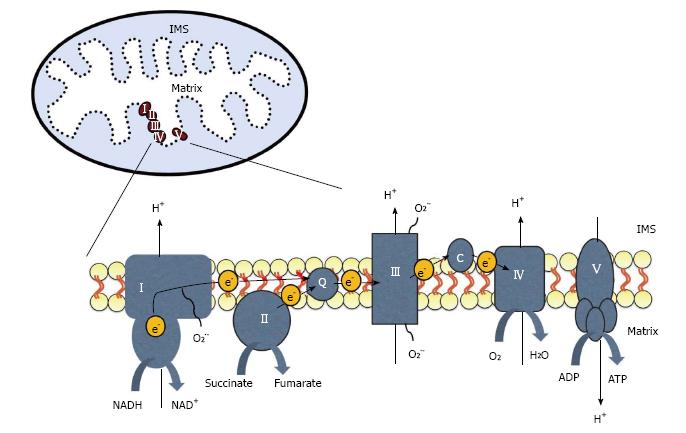
Figure 2 The electron transport chain is located on the inner mitochondrial membrane.
Electrons (e-) enter through complex I or II and are transferred to complex III by coenzyme Q (Q). Electrons then move between complex III and IV by cytochrome c (c). Electrons can leak from the chain and react with oxygen to create superoxide (O2-). The movement of electrons is coupled to the pumping of protons (H+) from the mitochondrial matrix into the intermembrane space (IMS). This gradient is then used by ATP synthase, or complex V, to generate ATP.
The main source of electrons for the ETC is the reduced form of nicotinamide adenine dinucleotide (NADH) produced by the citric acid (Kreb’s) cycle and the oxidation of fatty acids (β-oxidation; Figure 1). Another source of electrons is succinate, a byproduct of the Kreb’s cycle. Electrons enter the ETC through complex I (NADH dehydrogenase) or complex II (succinate dehydrogenase) and are then transferred to complex III (cytochrome bc1 complex) through a lipid soluble carrier molecule, coenzyme Q (Figure 2). Electrons then move between complex III and IV by way of a water soluble carrier molecule, cytochrome c. Molecular oxygen is reduced and water is produced by complex IV, cytochrome c oxidase. The movement of electrons through the ETC is coupled to ATP production by ATP synthase, or complex V. This protein complex converts ADP to ATP and is coupled to proton movement from the IMS back into the matrix. This process of synthesizing ATP in the mitochondria is called oxidative phosphorylation.
Electrons can leak from the ETC and react with molecular oxygen to create ROS like O2- (Figure 2). Complex I and III are the primary sites of electron leak and are sensitive to ROS injury[11]. ROS play an important role in skeletal muscle plasticity and activate many signaling cascades including increasing peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), the master regulator of mitochondrial biogenesis[12,13]. Cells have antioxidants in the cytoplasm and mitochondrial matrix in order to neutralize ROS. However, if the balance between antioxidants and ROS is disturbed, large amounts of ROS can result in the oxidation of proteins, lipids and DNA. As discussed above, access across the IMM is tightly regulated and disruption of the electrochemical gradient across it results in decreased ATP synthesis and increased ROS production. In addition to mitochondrial ROS production, in skeletal muscle, superoxide is also produced in the sarcoplasmic reticulum, transverse tubules, sarcolemma, and the cytosol[14].
Mitochondrial biogenesis
Mitochondrial biogenesis includes transcription of genes by the nuclear and mitochondrial genomes. Mitochondrial DNA (mtDNA) is circular and encodes 13 proteins essential for ETC function. MtDNA is susceptible to damage by ROS because it is not protected by histones like nuclear DNA. Deletions in mtDNA are observed in several diseases and may result in a decrease in gene expression of mitochondrial encoded genes important for ETC function, resulting in increased ROS production and decreased ATP production.
Mitochondrial biogenesis is regulated, in part, by the master regulator PGC-1α and varies based on the energy needs of the cell. After translation and mitochondrial import, proteins of the ETC are assembled into protein complexes and generate ATP through oxidative phosphorylation[13].
Mitochondrial dynamics
Remodeling of mitochondria occurs by fission and fusion. Fusion of mitochondria results in a large mitochondrial network, while fission pinches off a part of this network. After fission, mitochondrial fragments can be tagged for degradation by a specialized type of autophagy, termed mitophagy, or they can rejoin the mitochondrial network by fusion. When there is a scarcity of nutrients the mitochondrial network is fused in order to increase mitochondrial bioenergetic efficiency, while an abundance of nutrients results in a fragmented mitochondrial network[15]. Mitochondria also make contact with other subcellular organelles, including the endoplasmic reticulum. These endoplasmic reticulum mitochondria-associated membranes play an important role in cellular functions including mitochondrial division, apoptosis, and lipid and calcium regulation[15,16].
METHODS FOR STUDYING MITOCHONDRIA
There are multiple techniques that can be used to measure mitochondrial dynamics and function. These include measuring mRNA or protein expression, enzyme activity, and oxygen consumption by respirometry. Skeletal muscle biopsies are most commonly used in human studies since spinal cord and brain tissue is inaccessible.
mRNA and protein expression
mRNA and protein expression of molecules involved in mitochondrial biogenesis, remodeling and degradation can be observed by quantitative (real-time) polymerase chain reaction (qPCR) and western blot. This is the easiest way to detect changes in cellular signaling cascades and allows for the elucidation of where in the cascade there is a potential defect caused by disease or injury. Western blot analysis of ETC proteins and other mitochondrial proteins can be assayed as an estimate of respiratory chain function and mitochondrial mass, respectively. However, this type of analysis assumes that the proteins will then be imported into the mitochondria and properly assembled into the ETC complexes. There is also the assumption that a change in the expression of one protein from each complex is representative of the whole complex when in fact there are multiple proteins in each complex. For example, the mammalian complex I contains 45 different proteins[17].
Enzyme activity
One way to estimate mitochondrial function is to measure the activity of individual ETC complexes using a spectrophotometer. This can be done by measuring specific donor-acceptor oxidoreductase activities as previously described[18,19]. By using specific substrates and inhibitors each complex can be assayed individually. Linked activity between complexes can be measured by adding either NADH (for complex I) or succinate (for complex II) and measuring the reduction of cytochrome c by complex III[18,19]. Other key enzymes of the citric acid cycle (i.e., citrate synthase) and β-oxidation pathways [i.e., β-hydroxyacyl-CoA dehydrogenase (β-HAD)] can also be measured spectrophotometrically[18,20].
The benefit of spectrophotometric analysis is that it uses a small amount of tissue, samples can be previously frozen, and spectrophotometers are common lab equipment[19]. A limitation of spectrophotometric analysis is that it shows maximum capacity of individual complexes and does not necessarily represent physiological function. However, with the limited sample size obtained by human skeletal muscle biopsy and the need by many labs to freeze tissue, this may be a good option for many research groups.
Previous research has used human biopsy or autopsy specimens from tissues such as skeletal muscle, liver and skin[21]. Studies have been conducted using both tissue homogenates and isolated mitochondria from previously frozen human skeletal muscle[22,23]. Isolating mitochondria is ideal for understanding mechanism; however, the cellular context is lost. Also, more tissue is needed compared to running tissue homogenates because some mitochondria will be lost in the isolation process. A benefit of analyzing tissue homogenates is that the mitochondria remain in their cellular context, giving a more physiological reading. A limitation of analyzing tissue homogenates is the high non-mitochondrial NADH oxidase activity of some tissues[24].
Respirometry
Measuring oxygen consumption of isolated mitochondria or permeabilized cells by respirometry is currently the gold standard for assaying mitochondrial function. Respiration shows that the ETC is functioning because oxygen is necessary for ATP synthesis. The addition of substrates and inhibitors allows assessment of individual ETC complexes and coupling to ATP synthesis. Respiration can determine mitochondrial dysfunction not identified by spectrophotometric analysis, including impairment in mitochondrial membrane transport, problems with substrate utilization, and defects in fatty acid metabolism[25]. Protocols have recently been developed to analyze mitochondrial function from as little as 20-50 mg of muscle tissue[26]. However, this technique is labor intensive and samples cannot be previously frozen because freezing uncouples the ETC from ATP synthesis.
CHANGES IN BODY COMPOSITION AND METABOLISM AFTER SCI
SCI is usually a result of trauma to the spine, resulting in damage to cells that send messages to and from the brain. Damage can be to the upper motor neurons that project from the brain to the spinal cord or lower motor neurons that project from the spinal cord to the muscles. The location and severity of the injury largely determines the extent of impairment. Injuries resulting in motor and sensory impairment distal to the level of injury are classified as either complete or incomplete SCI, with incomplete injury resulting in spared sensation and/or motor function. The loss of peripheral nervous system control below the level of injury results in decreased mobility. This immobility, combined with hormonal changes and poor dietary habits result in decreased muscle mass and increased adipose deposition[6,7,27]. These changes put individuals with SCI at a high risk for developing cardiovascular disease, type II diabetes, and obesity[7,28,29]. Recent studies have shown a link between the deterioration in body composition and the impaired metabolic profile after SCI[30,31]. However, there are still many questions that remain unanswered at the cellular level.
Body composition after SCI
Drastic changes in body composition follow SCI[8]. Skeletal muscle atrophy combined with inactivity and poor diet contributes to the increased prevalence of obesity in this population[32]. Excess body fat, particularly around the waist, is a risk factor for a number of conditions including cardiovascular and metabolic disease[31,33]. Measurements of body mass index (BMI) do not take into account regional distribution of adipose tissue and underestimate fat mass in persons with SCI[34]. Waist circumference measurements do not distinguish between subcutaneous adipose tissue and ectopic [visceral adipose tissue (VAT)]. It is important to distinguish between these two adipose tissue types, as an increase in VAT is a risk factor for cardiovascular and metabolic disease[35]. Waist circumference measurements underestimate the amount of VAT in individuals with SCI. Increased waist circumference is correlated with the amount of VAT in able bodied (AB) individuals but this is not the case in the SCI population[36]. SCI individuals have more VAT than AB individuals with the same waist circumference[37]. Collectively, these data suggest that there adipose tissue deposition is increased after SCI and that the distribution is altered compared to AB individuals. For a review on this topic, see[8].
In addition to increased adipose tissue disposition, individuals with SCI experience significant changes to their skeletal muscle. These changes include significant muscle atrophy, conversion of muscle fiber type from oxidative to fast glycolytic, and an increase in intramuscular fat (IMF). Both complete and incomplete SCI results in substantial atrophy of muscles below the level of injury. Incomplete SCI resulted in a 33% decrease in thigh muscle cross sectional area and an increase in IMF six weeks post-injury compared to AB controls[32]. The conversion of muscle fiber type to fast glycolytic results in an quickly fatigued muscle that can be damaged easily[38]. Additionally, an increase in fast glycolytic muscle fibers decreases insulin sensitivity and may lead to diabetes[39].
As discussed above, increased VAT, but not subcutaneous adipose tissue, is a risk factor for cardiovascular disease, glucose intolerance, insulin resistance, and hyperlipidemia[35]. This may be due to the infiltration of immune cells into VAT and subsequent secretion of inflammatory cytokines including tumor necrosis factor α (TNFα), interleukin-1β (IL-1β) and IL-6. Previous research suggests that inflammatory cytokines released by adipose tissue accelerate skeletal muscle atrophy[40-42]. A recent study investigating the interactions between adipose tissue and skeletal muscle revealed that VAT adipocytes from obese subjects decreased cultured myotube thickness and resulted in a gene expression profile suggestive of muscle atrophy[41]. The proposed mechanism is through the release of IL-1β and IL-6 and decreased insulin growth factor signaling, resulting in insulin resistance. Another type of adipose tissue that is similar to VAT, IMF, is increased after SCI and may be a contributing factor to the development of insulin resistance[32,43]. The mechanisms underlying the interplay between adipose tissue and skeletal muscle are just beginning to be understood.
Metabolism after SCI
In addition to changes in body composition, metabolism is disrupted after SCI. As many as 55% of individuals with SCI have metabolic syndrome, which is characterized by three or more of the following conditions: Obesity, high blood pressure, insulin resistance, high triglycerides, and low high-density lipoprotein (HDL) cholesterol levels[44]. Impaired glucose tolerance was observed in 56% of persons with SCI, compared with only 18% of AB controls[45]. Individuals with SCI also have increased low-density lipoprotein (LDL) cholesterol[46,47]. These conditions worsen with age and put individuals at risk for developing cardiovascular disease and type II diabetes.
MITOCHONDRIAL HEALTH STATUS AFTER SCI
CNS mitochondrial health after SCI
The immediate damage to the spinal cord, including damage to axons and cells at the injury site is called primary injury. Models of SCI have shown an increase in intracellular sodium, chloride and calcium 15-60 min after injury[48]. An increase in intracellular calcium may result in apoptosis if the excess calcium taken up by mitochondria triggers mitochondrial permeability transition pore opening. Following this initial insult is a secondary injury, characterized by invasion of inflammatory cells and more cell death as cells invade not only the injury site, but also the spared nervous tissue. Neuronal death leads to loss of motor or sensory function and loss of oligodendrocytes leads to axonal demyelination[49].
Mitochondrial respiration in the spinal cord through complexes I and II is decreased and oxidative stress is increased at 12 and 24 h, but not after 6 h after SCI in a rat model[50]. In another study respiration and complex I and IV enzyme activity was decreased in the spinal cord after SCI[51]. In this study mitochondrial function was improved by treatment with an antioxidant. Complex I, complex IV, and pyruvate dehydrogenase are mitochondrial enzymes that are particularly vulnerable to damage by ROS and are decreased after SCI[52]. Decreasing ROS or increasing function of these enzymes may improve functional outcomes after SCI.
Skeletal muscle mitochondrial health after SCI
There is limited knowledge about the changes in mitochondrial function following SCI in humans. However, indirect evidence of mitochondrial function using near-infrared resonance spectroscopy to measure tissue oxygenation revealed that muscle oxidative capacity was decreased 50%-60% in participants with SCI 2.7-22 years after injury compared to AB controls[53]. A similar deficit was observed using histochemistry to measure succinate dehydrogenase (SDH) activity in muscle biopsy samples from paralyzed muscle 2-11 years post injury compared to AB controls[54,55]. In contrast, a study analyzing SDH and GAPDH activity, markers of complex II and glycolytic capacity, respectively, 6-24 wk after injury found increased activity despite greater fatigability of muscles[38]. The reason for these discrepant results in unclear, but it could be that early after injury muscle atrophy and fiber type changes results in a compensatory increase in oxidative and glycolytic enzymes but long periods of muscle inactivity result in reduced activity of oxidative and glycolytic pathways[38,54,55]. More research is needed to determine the effect of SCI on mitochondrial function.
Mitochondria are also dysfunctional in a number of metabolic diseases including type II diabetes and obesity. A large network of fused mitochondria is observed in healthy skeletal muscle, while muscle from obese and type II diabetics is fragmented[56]. Skeletal muscle mitochondrial function is decreased as well, with a 2-3 fold decrease in NADH oxidase (complex I) activity normalized to mitochondrial content in obese and type II diabetics compared to control[57].
Exercise interventions have been shown to increase skeletal muscle mitochondrial function and improve insulin sensitivity in obesity, diabetes, and aging[58,59]. Some options for exercise intervention after SCI include neuromuscular electrical stimulation-induced weight lifting and functional electrical stimulation (FES) cycling. Sixteen weeks of electrical stimulation-induced resistance training increased muscle mass and improved mitochondrial function by 25% in patients with SCI[60]. FES cycling has also been shown to increase mitochondrial function in patients with SCI. Eight weeks of FES cycling resulted in an increase in citrate synthase, a marker of mitochondrial mass[61]. Similarly, studies found increased citrate synthase as well as increased function of enzymes involved in glycolysis and β-oxidation[62,63]. Finally, SDH was increased after 4 wk of training, suggesting that complex II activity is increased with exercise[64]. Similarly, a recent study showed that a single session of low frequency electrical stimulation increased genes involved in muscle metabolism, including PGC-1α[65]. Collectively, these studies suggest that paralyzed skeletal muscle is malleable and can increase mitochondrial function in response to exercise. Additionally, IMF has been shown to decrease after resistance exercise training[66]. This would provide additional benefit to skeletal muscle and may improve insulin sensitivity.
SIGNIFICANCE AND FUTURE DIRECTIONS
Mitochondria are vital for energy production and play a role in a number of cellular processes including cell signaling, cell cycle progression, calcium regulation and cell death. These organelles are dynamic, and undergo changes in activity and number in response to cellular energy needs. A decrease in neuron and skeletal muscle mitochondrial function is observed in a number of disease and injury states including CNS trauma, neurodegenerative disease, type II diabetes and obesity[1-3]. However, we know very little about mitochondrial function in patients with chronic SCI. We are just beginning to understand the role of mitochondria in insulin resistance and how skeletal muscle mitochondrial function is disrupted in patients with SCI. Future research needs to be done using functional assays to assess activity of individual ETC complexes, as well as its coupling to ATP synthesis.
Increasing mitochondrial function by pharmacological activation of mitochondrial biogenesis is an active area of research[67]. There are a number of FDA approved medications as well as naturally occurring substances that activate mitochondrial biogenesis. For example, resveratrol, which is found in red wine, activates sirtuin 1 (SIRT1) and increases PGC-1α activity and mitochondrial function and was shown to improve insulin resistance in diabetic patients[68,69]. Small molecules that activate SIRT1 with improved bioavailability and potency have been developed and are currently being tested in humans. FDA approved pharmacological activators of mitochondrial biogenesis include the β2-adrenergic receptor agonist formoterol[70], the anti-diabetic drug metformin[71], the phosphodiesterase inhibitor sildenafil[72], the PPARγ agonist rosiglitazone[73], the mitochondrial permeability transition pore inhibitor cyclosporine A[74], and the angiotensin-converting enzyme inhibitor captropril[75], among others. Although these compounds are thought to exert their effects at least in part by increasing mitochondrial biogenesis, there are currently no specific activators of mitochondrial biogenesis. Future studies need to investigate the safety and efficacy of systemically increasing mitochondrial biogenesis, as well as optimizing dosing in order to maximize the therapeutic benefit.
In order to study mitochondrial function after disease or injury or to assess the efficacy of mitochondrial targeted therapies, skeletal muscle biopsies could be used because of the inaccessibility of the brain and spinal cord in humans. However, recent studies have suggested that the bioenergetic profile of blood cells is associated with physical function and inflammation as well[76,77]. Indeed, mitochondrial dysfunction is seen in blood from patients with a number of diseases including neurodegenerative diseases and type II diabetes[78,79]. Peripheral blood mononuclear cells from patients with type II diabetes and chronic kidney disease have increased inflammatory cytokines, decreased mitochondrial function and increased ROS production[80]. These studies suggest that blood cell bioenergetics may predict systemic mitochondrial function and may act as biomarkers for metabolic stress and surrogate markers for the severity of disease progression and the efficacy of therapeutics[80,81]. This represents an intriguing possibility, as obtaining blood samples are much less invasive than biopsies and could be taken more frequently in order to better characterize the time course of therapeutic intervention.
There are a number of different techniques for analyzing cellular signaling pathways and mitochondrial function. Researchers should carefully weigh the convenience of non-invasive techniques with the mechanistic detail provided by analyzing biopsy tissue. If choosing to analyze biopsy tissue, care should be taken to obtain the proper amount of tissue required for the assay and to prepare it properly in order to preserve mitochondrial function. For samples that need to be frozen, spectrophotometric analysis may be the best option for analyzing mitochondrial function, while respiration will be ideal for fresh tissue samples. Another research consideration is whether or not to isolate mitochondria and this may depend on the sample size.
As discussed above, both resistance training and FES cycling has been shown to increase mitochondrial function in persons with SCI. In addition, electrical stimulation-induced resistance training reduced VAT and IMF and increased insulin sensitivity while increasing muscle mass[66]. FES cycling has been shown to improve insulin sensitivity as well, but the effect on muscle size and body composition were minimal to modest[82]. It is unknown if conditioning the muscles with resistance training prior to FES cycling would result in greater mitochondrial and metabolic outcomes.
CONCLUSION
There is limited knowledge regarding skeletal muscle mitochondrial health following SCI. Challenges may stem from difficulties in capturing muscle biopsies and running biochemical analysis to determine mitochondrial mass or activity by spectroscopy or respiration. Non-invasive procedures like near-infrared resonance spectroscopy may reflect mitochondrial activity; however, mechanistic dysfunctions inclividual of complexes may be limited.
A better understanding of how mitochondrial function is impacted in patients with chronic SCI is critical for developing interventions to increase mitochondrial function and improve metabolic outcomes. Skeletal muscle or blood cell bioenergetics may predict overall mitochondrial health and therefore be a surrogate marker of disease progression and treatment efficacy. Increasing mitochondrial function immediately following SCI may decrease cell death and improve functional outcomes. Improvement in mitochondrial function by exercise or pharmacological interventions in chronic SCI may decrease comorbidities. This will result in better health for patients and a lower financial burden for their health care. A better understanding of mitochondrial biology may also translate to a number of other diseases in which mitochondrial are dysfunctional, particularly insulin resistance, type II diabetes, and obesity.
ACKNOWLEDGMENTS
We would like to thank the Hunter Holmes McGuire VA Medical Center and Virginia Commonwealth University for providing the environment to conduct clinical research.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Berra LV, Rabchevsky AG S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ