Copyright
©2013 Baishideng Publishing Group Co.
World J Orthop. Oct 18, 2013; 4(4): 207-217
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.207
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.207
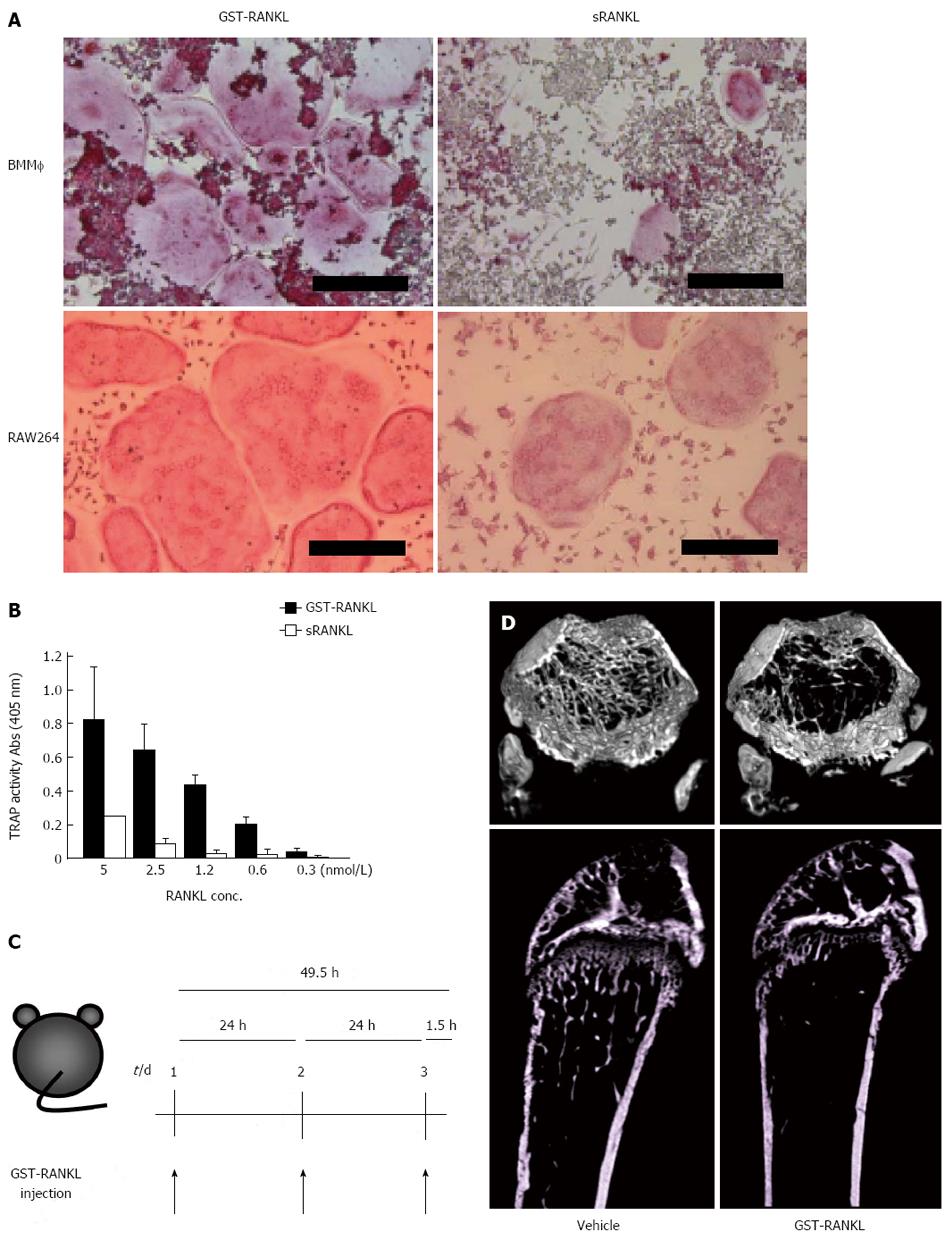
Figure 2 Establishment of GST-RANKL-injected bone loss model.
A: Osteoclasts were generated with 5 nmol/L glutathione-S transferase-receptor activator of nuclear factor-κB ligand (GST-RANKL) or sRANKL from bone marrow macrophages (BMM) and RAW264 cells in the presence and absence of M-CSF, respectively. The cells were fixed and stained for tartarate-resistant acid phosphatase (TRAP). Bars = 250 m; B: Activities of GST-RANKL and sRANKL were compared using in vitro osteoclastogenesis assay with RAW264 cells by TRAP solution assay. The TRAP activity represents the number of osteoclasts. C: Experimental design. PBS (vehicle) or GST-RANKL was injected intraperitoneally at 24-h intervals for 3 d into 7-wk-old female mice. Mice were sacrificed 90 min after the last injection; D: Micro computer tomography 3D images of femurs of mice treated with vehicle or 2 mg/kg GST-RANKL. The upper and lower panels are 3D cross-sectional and longitudinal images, respectively.
- Citation: Yasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World J Orthop 2013; 4(4): 207-217
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/207.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.207