Published online Sep 18, 2012. doi: 10.5312/wjo.v3.i9.142
Revised: August 21, 2012
Accepted: September 15, 2012
Published online: September 18, 2012
The interaction between the receptor activator of NF-κB ligand (RANKL) and its receptor RANK plays a critical role in the development and function of diverse tissues. This review summarizes the studies regarding the functions of RANKL signaling in immune regulatory systems. Previous in vitro and in vivo studies have indicated that the RANKL signal promotes the survival of dendritic cells (DCs), thereby activating the immune response. In addition, RANKL signaling to DCs in the body surface barriers controls self-tolerance and oral-tolerance through regulatory T cell functions. In addition to regulating DC functions, the RANKL and RANK interaction is critical for the development and organization of several lymphoid organs. The RANKL signal initiates the formation of clusters of lymphoid tissue inducer cells, which is crucial for lymph node organogenesis. Moreover, the RANKL-RANK interaction controls the differentiation of M cells, specialized epithelial cells in mucosal tissues, that take up and transcytose antigen particles to control the immune response to pathogens or commensal bacterium. The development of epithelial cells localized in the thymic medulla (mTECs) is also regulated by the RANKL-RANK signal. Given that the unique property of mTECs to express a wide variety of tissue-specific self-antigens is critical for the elimination of self-antigen reactive T cells in the thymus, the RANKL-RANK interaction contributes to the suppression of autoimmunity. Future studies on the roles of the RANKL-RANK system in immune regulatory functions would be informative for the development and application of inhibitors of RANKL signaling for disease treatment.
- Citation: Akiyama T, Shinzawa M, Akiyama N. RANKL-RANK interaction in immune regulatory systems. World J Orthop 2012; 3(9): 142-150
- URL: https://www.wjgnet.com/2218-5836/full/v3/i9/142.htm
- DOI: https://dx.doi.org/10.5312/wjo.v3.i9.142
The tumor necrosis factor (TNF) family member receptor activator of NF-κB ligand (RANKL) and its receptor RANK regulate diverse physiological functions and organ development in the body. Because RANKL- and RANK-deficient mice exhibit severe osteopetrosis, multiple studies have focused on the role of the RANKL-RANK axis and its underlying mechanism in the regulation of bone homeostasis through osteoclast differentiation. As a result of these extensive studies, a fully humanized anti-RANKL neutralizing antibody has been developed and recently approved for the treatment of osteoporosis, rheumatoid arthritis and cancer bone metastasis. However, RANKL and RANK were originally identified as a cytokine and its receptor, respectively, that control the function of dendritic cells (DCs)[1,2] in addition to their role in osteoclast differentiation[3]. Moreover, RANKL- and RANK-deficient mice (RANKL-KO and RANK-KO) manifest defects in the development, organization and function of several lymphoid organs[2,4-6]. In this review, we summarize the current knowledge regarding the roles of RANKL/RANK signaling in immune regulatory systems, including immune activation, immunological tolerance and lymphoid organogenesis.
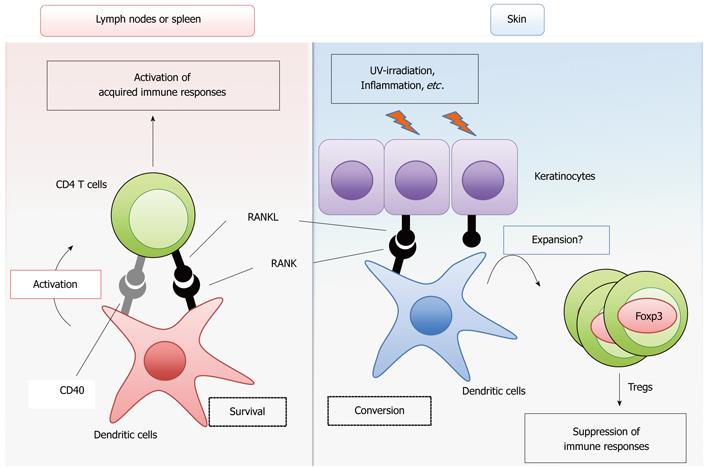
RANK shows a relatively high homology with CD40 among the TNF receptor family members[2]. The cytoplasmic region of RANK contains binding sites for the tumor necrosis factor receptor-associated factor (TRAF) family proteins TRAF2, 3, 5 and 6[7-10], which is a structural property shared with CD40[11]. These TRAF family proteins mediate the activation of the NF-κB and MAPK pathways to result in the transcriptional activation of genes required for proliferation, survival and differentiation[7,11]. Therefore, it is reasonable to assume that RANK signaling initiates cellular responses similar to CD40 signaling by triggering these common intracellular signaling pathways. A large number of studies have revealed that CD40 signaling induces the maturation, activation and survival of DCs[12,13]. DCs are classified into several subsets according to their functions, localizations and cell surface markers[14]. Conventional DCs (cDCs) are the prototypical professional antigen-presenting cells that engulf, process and present antigens, thereby priming and activating T cells for the initiation of the acquired immune response. The RANK cDNA was discovered by the direct sequencing of a cDNA library prepared from cDCs. In addition, RANKL expression is upregulated in activated T cells[15]. Therefore, early studies analyzed the effects of RANKL treatment on the ability of cDCs to prime T cells in vitro. Indeed, two independent studies revealed that RANKL treatment promotes the survival of cDCs, thereby efficiently priming T cells[2,16] (Figure 1). Interestingly, whereas CD40 signaling upregulates the surface expression of the co-stimulatory molecules CD80/86 and the major histocompatibility complex (MHC) molecules, RANK signaling does not[2,16]. Thus, although RANK and CD40 activate similar intracellular signaling pathways, the outputs of these two signals in the cells are slightly different. The “adjuvant” effect of the RANKL signal was further confirmed by ex vivo transfer of cDCs treated with RANKL[17]. The impact of RANK deficiency on DC function has not been clearly defined in RANKL-KO and RANK-KO mice, most likely because these null mutant mice manifest complicated and combined phenotypes and die at young ages after birth. However, it has been shown that blocking the RANKL-RANK interaction by injecting non-membrane-bound RANK-Fc protein suppresses CD4+ T cell-mediated immune activation induced by viral[18] and parasitic infections[19] in CD40-deficient (CD40-KO) mice, suggesting that the CD40 signal compensates for the lack of RANK signaling in CD4+ T cell priming by cDCs. Moreover, the role of the RANKL signal in the in vivo immune response was investigated in a spontaneous autoimmune disease model induced by the lack of IL-2[20]. The T cell-mediated intestinal inflammation in IL-2 deficient mice was significantly mitigated by the administration of osteoprotegerin (OPG)[21], a natural decoy receptor for RANKL[22,23]. Furthermore, the increase in the numbers of activated cDCs in the intestine of IL-2 deficient mice was significantly suppressed when the mice were treated with OPG. Thus, this study suggested that the RANKL-mediated survival of cDC promotes autoimmune inflammatory bowel disease in vivo. These studies support the idea that RANKL signaling activates the immune response by promoting the survival of cDCs, enhancing T-cell priming and activation. In contrast to these findings, the overexpression of OPG in rodents did not cause obvious changes in the innate or acquired immune response[24,25]. Overall, it is likely that the activation of the immune response by RANKL signaling occurs only in specific circumstances or redundantly with other cytokine signals, such as CD40.
It is well recognized that the immune regulatory functions of DCs are dependent on the DC subtype and maturational stage[14]. Studies have been undertaken to investigate the role of RANKL signaling in the functions of DCs localized to the surface barriers of the body, such as the mucosal tissues and the skin. RANKL was shown to exhibit an immunosuppressive effect through these DCs (Figure 1). In the skin, the RANKL signal altered the function of the epidermal dendritic cells, increasing the number of Foxp3-positive regulatory T cells (Tregs)[26], a CD4+ helper T cell subset critical for suppressing autoimmune responses and excess immune reactions in the body[27]. The expression of RANKL is upregulated in skin keratinocytes by ultraviolet light irradiation or inflammation[26] possibly mediated by the activity of prostaglandin E2[28]. Thus, stimulation of epidermal DCs with RANKL induces systemic immunosuppressive activity. In contrast to the effect of RANKL, the enforced expression of CD40L in keratinocytes induces severe autoimmune disease in the skin[26,29,30]. Interestingly, the autoimmune disease provoked by the enforced expression of CD40L is significantly suppressed by the enforced expression of RANKL in keratinocytes[26]. Thus, RANKL and CD40 signaling result in diametrically different immune responses in the skin. Moreover, in contrast to the autoimmune intestine disease model induced by IL-2 deficiency, which is described above[21], two studies suggest the immunosuppressive effects of RANKL signaling in the intestines[31,32]. Peyer’s patches are lymphoid tissues located in the intestine that control the immune response to foods or commensal bacteria. The stimulation of Peyer’s patch-derived cDCs with RANKL increased the expression of IL-10[31], a cytokine with anti-inflammatory activity[33]. Consistently, the treatment of mice with RANKL enhanced the oral tolerance to an ovalbumin challenge[31]. In another study, a model of colitis induced by the transfer of the CD4+CD45RBhigh T cell fraction into lymphopenic mice was utilized as a T cell-dependent autoimmune disease model[34]. In this model, colitis is suppressed by the simultaneous transfer of the CD4+CD25+ T cell fraction, which is enriched for Foxp3+-regulatory T cells (Tregs)[34]. It was demonstrated that the administration of an anti-RANKL neutralizing antibody inhibits the suppression of colitis by the transfer of CD4+CD25+ T cells[32], suggesting that RANKL supports the function of the CD4+CD25+ regulatory T cells in the intestine. These data suggest that RANKL-mediated modulation of the surface barrier DCs results in the suppression of autoimmune responses and detrimental immune responses toward innocuous foreign antigens derived from foods or commensal bacteria in the intestines.
The lymphoid organs can be classified into primary lymphoid organs and secondary lymphoid organs. The primary lymphoid organs, the bone marrow and thymus, primarily provide the environment that is required for the differentiation and development of immune cells. The secondary lymphoid organs, including the lymph nodes, spleen and Peyer’s patches, are tissues that trap antigens and initiate the acquired immune response. RANKL signaling is involved in the development and organization of these organs. Although the bone marrow is a primary lymphoid organ whose environment is regulated by RANKL, it is reviewed elsewhere in this issue[35] and is not further discussed here. Previous studies have revealed that RANKL- and RANK-KO mice lack lymph nodes[4-6]. Lymph nodes consist of several different types of cells, including T cells and B cells, DCs, reticular cells, stromal cells and specialized endothelial cells[36]. Communication between the lymphoid cells and the stromal cells is required for the formation and maintenance of the lymph node structure. Stromal cells express and secrete the chemokines CXCL13, CCL19 and CCL21 in the defined region of the lymph nodes, which are necessary for the accumulation and retention of B cells and T cells in distinct areas of the lymph nodes[37-39]. These lymphocytes in turn express cytokines that induce the expression and secretion of these chemokines from the stromal cells.
RANKL signaling controls the organogenesis of fetal lymph nodes. The early development of a lymph node begins with the movement of lymphoid tissue inducer cells (LTi), a unique cell subset derived from the fetal liver, into the region where the lymph nodes will develop[39-41]. The LTi interact with the resident stromal cells to initiate colonization and cluster formation, thereby forming lymph node anlages[39-41]. The TNF family members, lymphotoxinα (Ltα) and membrane-bound lymphotoxinβ (Ltβ), which are secreted by the LTi, are essential for lymph node organogenesis. Ltα and Ltβ form heterotrimers on the cell surface of LTi, which bind to the lymphotoxinβ receptor (LtβR) on the stromal cells. This interaction induces the expressions of chemokines and adhesion molecules by the stromal cells to attract and retain LTi. A complex pattern of RANKL expression has been detected during the process of early lymph node development[42,43]. RANKL is initially expressed in the LTi[42,43], which is independent of LtβR signaling[42]. Subsequently, lymphoid tissue organizer cells derived from mesenchymal stromal cells initiate the expression of RANKL in a LtβR signal-dependent manner[42]. A deficiency in RANKL signaling results in a severe reduction in the number of LTi[5], suggesting that the requirement for RANKL signaling begins during the initial LTi colonization process of early development. Interestingly, whereas RANKL- and RANK-KO null mice lack lymph nodes, the inhibition of RANKL signaling by the overexpression of its decoy receptor OPG did not impair the development of lymph nodes[24,25], implying that very small quantities and the temporary expression of RANKL are sufficient for the development of lymph nodes. Notably, whereas LtβR signaling is also essential for the development of Peyer’s patches, which are mucosal lymphoid organs located in the intestines, RANKL-KO mice contain Peyer’s patches[4,5]. Consistently, TRAF6, a critical signal transducer of RANK signaling, is required for lymph node development but not for the development of Peyer’s patches[44,45]. Thus, the RANKL-RANK-TRAF6 axis is dispensable for the formation of Peyer’s patches. Moreover, the formation of the splenic architecture is regulated by TNF family signals and chemokine signals[46]. However, the splenic architecture is not practically impaired by the absence of RANK signaling[4,5].
Interestingly, although RANKL is not essential for the formation of Peyer’s patches, the expression of RANKL was detected in the stromal cells of Peyer’s patches and other lymphoid tissues of the intestine[47]. Moreover, the Peyer’s patches of RANKL-KO mice are smaller than in control mice[4,5]. These observations imply that RANKL signaling may contribute to the formation of the microarchitecture of Peyer’s patches. Indeed, a recent study has uncovered a role for the RANK signal in the development of M cells[48], a specialized subset of epithelial cells found in mucosal lymphoid organs, such as Peyer’s patches and isolated lymphoid follicles[49]. M cells are localized to the epithelial cell layers that cover these tissues and they transcytose particulate foreign antigens and microorganisms from the lumen. This process is critical for controlling the immune response against pathogens and commensal flora[50]. RANKL-KO mice possessed fewer M cells in Peyer’s patches[48]. Moreover, the administration of an anti-RANKL neutralizing antibody reduced the number of M cells[48]. Thus, this study supported a novel role of RANKL signaling in the initiation of M cell development.
Thymic epithelial cells (TECs) are essential for the development and selection of T cells in the thymus[51,52]. Several recent studies have revealed that RANKL signaling promotes the development of a subset of TECs that are essential for preventing autoimmunity by eliminating self-reactive T cells in the thymus[53-55]. We briefly describe the fundamental knowledge regarding T cell selection and development in the thymus and its correlation with TECs. Then, we summarize the contribution of RANKL signaling to the development of TECs and to preventing autoimmunity.
A large portion of the T cells in the body develop in the thymus and developed T cells express widely diverse “repertoires” of T cell antigen receptors (TCR) with different antigen specificities. After the TCR is expressed on the cell surface, the T cell repertoires are shaped according to two aspects; the first is to recognize self-major histocompatibility complex (MHC) molecules and the second is to be unresponsive to self-antigens[56]. The achievement of this selectivity is explained by the so-called “avidity (affinity)” model[57,58]. The basic concept of this model is that the fate of each T cell is dependent on the avidity between its T cell antigen receptor (TCR) and complexes of self-antigen peptides and MHC molecules, which are presented by antigen-presenting cells in the thymus. T cells recognizing the self antigen-MHC complex with very low or high avidity through their TCRs undergo apoptosis and only the T cells recognizing the self antigen-MHC complex with “moderate” affinity can survive. As result, T cells incapable of binding to self-MHC and T cells responsive to self antigens in other tissues would be eliminated by this mechanism. Thus, this selection mechanism permits the generation of T cell repertoires that recognize foreign antigens that bind to self-MHC with high affinity.
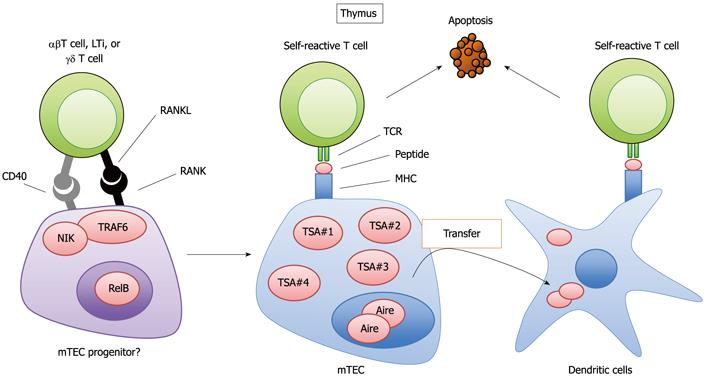
T cell selection is closely related to the development of T cells in the thymus. The thymus is anatomically divided into the cortex and the medulla[59,60]. Different developmental and selection processes occur in each region[59,60]. First, T lymphoid progenitor cells from the bone marrow develop into CD4 and CD8 double-positive immature T cells (CD4+CD8+ T cells) in the cortex. Subsequently, the CD4+CD8+ T cells interact with epithelial cells in the cortex (cTECs; cortical thymic epithelial cells), which are antigen-presenting cells expressing MHC molecules. Through this interaction, the CD4+CD8+ T cells that recognize the complex of self-antigen and MHC molecules presented by cTEC survive and successfully differentiate into CD4 or CD8 single-positive (CD4SP or CD8SP) T cells. The CD4SP or CD8SP T cells move to the medulla and are further scanned by the medullary thymic epithelial cells (mTECs). The mTECs have unusual properties with regards to gene expression; mTECs ectopically express a wide variety of self-antigens that are normally expressed in a tissue-restricted manner (TSAs, for example, insulin or caseins) (Figure 2)[61]. The ectopic expression of TSAs is in part regulated by the nuclear protein Aire (autoimmune regulator)[62,63], which suppresses the onset of autoimmune disease in humans and mice[63,64]. mTECs present TSAs to the medullary T cells directly or indirectly through the thymic dendritic cells (Figure 2)[65-67]. Those T cell repertoires that recognize TSA peptide with high avidity are eliminated (Figure 2); otherwise, they would initiate an immune response to the TSAs expressed in their cognate organs, potentially causing autoimmune disease. In fact, several lines of evidence have indicated that the dysregulation of this mechanism promotes autoimmune disease[63,68-71].
It is currently accepted that both mTECs and cTECs differentiate from common progenitor cells[72,73]. Moreover, evidence has suggested the presence of an mTEC progenitor[74]. However, the details of the developmental process from common progenitor to mature mTECs and cTECs are largely unknown. Several studies have revealed a role for RANKL signaling in the development of mature mTECs expressing Aire and TSAs[53-55] (Figure 2). The expression of RANK was detected in mTECs in the adult thymus[54,55] and in fetal thymic stroma organ cultures[54], which plausibly contain mTEC progenitors[53]. RANKL- and RANK-KO mice exhibited a partial defect in the development of mature mTECs[53-55]. The quantity of Aire-positive in mature mTECs is preferentially reduced by the lack of RANKL and RANK. Whereas mTEC levels are significantly reduced by the absence of RANK signals, a population of mature mTECs still exists. However, TRAF6 deficiency results in a severe defect in mTEC development compared to that in RANKL and RANK-deficient mice[68]. This difference can be explained by CD40-mediated compensation for RANK during TEC development, which is shown by the complete loss of mTECs in RANKL and CD40 double-deficient mice. Thus, RANK and CD40 cooperatively signal to promote the development of mTECs, which appears to be similar to the cooperation between RANK and CD40 in modulating the functions of cDCs[18].
The requirement for RANK signaling for the prevention of autoimmunity has also been tested. The transplantation of RANK-deficient fetal thymic stroma into nude mice results in inflammatory cell infiltration accompanied by the production of autoantibodies in the sera[53]. These data suggest that the lack of RANK in the thymic stromal cells is sufficient to induce autoimmunity. Another study demonstrated that the transfer of splenocytes from RANKL-KO mice into nude mice provokes mild autoimmunity[54]. Moreover, a much more severe autoimmune response was observed when splenocytes from RANKL and CD40 double-deficient mice were transferred into nude mice[54], which appeared to be correlated with the severe impairment in mTEC development in these doubly deficient mice. Consistently, mutant mouse lines lacking downstream molecules in the RANK signaling pathway (e.g., RelB-deficient mice or TRAF6-deficient mice) displayed severe phenotypes[68,75,76].
Several types of cells have been reported to express RANKL in the thymus. Interestingly, a previous study has revealed that LTi are localized to the embryonic thymus and provide RANKL for the embryonic development of Aire-expressing mTECs[53]. Moreover, the T cells that had newly differentiated from CD4+CD8+ T cells into CD4SP T cells were found to express high levels of RANKL[55]. A recent study has revealed that dendritic epidermal T cells (DETC), a subset of γδ T cells[77], express RANKL in the embryonic thymus[78]. In this study, it was also shown that RANKL signaling induced the expression of the immunoglobulin superfamily member Skint-1, which is involved in the selection of the DETC[79] that contribute to immune defense in the skin[77]. Thus, this study uncovered a novel connection between mTEC development and γδ T cell development in the fetal thymus mediated by the RANKL and RANK interaction.
The molecular mechanism underlying the expression of RANK in the mTEC progenitors remains unclear. A recent study has suggested that lymphotoxin beta receptor (LtβR) signaling is involved in the expression of RANK in immature mTECs[80]. This study suggested that RANK expression in TECs is under the control of LtβR signaling. However, it still remains unclear whether the LtβR signal upregulates the expression of RANK in individual TECs or promotes the proliferation and/or survival of RANK-expressing mTECs.
The interaction between RANKL and RANK induces activation of the NF-κB and the Mitogen-Activated Protein Kinase (MAPK) pathways[7]. During mTEC development, the NF-κB activation pathway appears to play a major role. Two major NF-κB activation pathways, the classical pathway and the non-classical pathway, are currently known[81]. The classical pathway induces the translocation of the RelA or c-Rel complex into the nucleus through the activation of the IκB kinase (IKK) complex containing IKKβ and NEMO. The activation of the non-classical pathway results in the translocation of RelB into the nucleus and requires the phosphorylation of IKKα by NF-κB inducing kinase (NIK). RANK signaling is capable of activating both pathways[82]. Interestingly, whereas both NF-kB activation pathways appear to be activated during mTEC and osteoclast development induced by RANKL, the contribution of each NF-κB pathway appears to differ between mTEC development and osteoclast differentiation. For instance, RelB-deficient (RelB-KO) mice, NIK-deficient mice and aly/aly mice, the last of which have a dysfunctional mutation in the NIK gene, exhibited normal or very mildly impaired osteoclastogenesis[82-86] and this pathway appears to be critical only in pathological osteolysis[82,83]. However, mTEC development was completely abolished in aly/aly and RelB-deficient mice[75,76,87]. Moreover, IKKα-deficient fetal thymic stroma did not differentiate into mature mTECs when transplanted onto the kidney[88]. In an in vitro study, RANKL-dependent maturation of mTEC was not detected in fetal thymic stroma prepared from aly/aly mice[54]. Moreover, RANKL signaling induces the nuclear localization of RelB in mature mTECs in fetal thymic stroma[54]. Thus, these data suggest that, in contrast to its involvement in osteoclast development, the non-classical NF-κB activation pathway is essential for the RANKL-dependent development of mTECs. The contribution of the classical NF-κB pathway to mTEC development has been suggested by the finding that TRAF6 is essential for the development of mTECs[54,68] as TRAF6 activates the classical NF-κB pathway and is dispensable for the non-classical NF-κB pathway[89]. Whereas c-Fos and NF-ATc1 are critical regulators of RANKL-mediated osteoclast development, the involvement of these genes in mTEC development has not been reported. These data suggest that RANKL signaling during mTEC development activates signal transduction pathways that are distinct from those activated during osteoclast development.
A humanized anti-RANKL antibody has been approved for the treatment of osteoporosis in postmenopausal women and cancer bone metastasis. Detailed studies in mouse models have clearly demonstrated the involvement of RANKL signaling in the functions of immune regulatory cells, such as dendritic cells, M cells and mTECs. Notably, the functions of dendritic cells and the maintenance of M cell numbers were impaired by the inhibition of RANKL signaling in adult mice. These results may be informative in applications of the anti-RANKL antibody for human treatments. Moreover, these relatively new findings could open the possibility of utilizing the anti-RANKL antibody for other applications by regulating the immune response.
Peer reviewers: Patrizia D Amelio, MD, PhD, Department of Surgical and Medical Disciplines, Section of Gerontology-University of Torino, Cso AM Dogliotti 14, 10126 Torino, Italy; Shang-You Yang, Associate Professor, Department of Biological Sciences, Wichita State University, 1845 Fairmount St., Wichita, KS 67260, United States
S- Editor Huang XZ L- Editor Roemmele A E- Editor Zhang DN
| 1. | Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190-25194. [PubMed] [Cited in This Article: ] |
| 2. | Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1712] [Cited by in RCA: 1639] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 3. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597-3602. [PubMed] [Cited in This Article: ] |
| 4. | Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2595] [Cited by in RCA: 2477] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 5. | Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467-1478. [PubMed] [Cited in This Article: ] |
| 6. | Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412-2424. [PubMed] [Cited in This Article: ] |
| 7. | Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998;273:20551-20555. [PubMed] [Cited in This Article: ] |
| 8. | Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 1998;273:28355-28359. [PubMed] [Cited in This Article: ] |
| 9. | Galibert L, Tometsko ME, Anderson DM, Cosman D, Dougall WC. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. J Biol Chem. 1998;273:34120-34127. [PubMed] [Cited in This Article: ] |
| 10. | Darnay BG, Besse A, Poblenz AT, Lamothe B, Jacoby JJ. TRAFs in RANK signaling. Adv Exp Med Biol. 2007;597:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131-151. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in RCA: 511] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265-272. [PubMed] [Cited in This Article: ] |
| 14. | Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12:101-113. [PubMed] [Cited in This Article: ] |
| 15. | Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162:2562-2568. [PubMed] [Cited in This Article: ] |
| 16. | Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075-2080. [PubMed] [Cited in This Article: ] |
| 17. | Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med. 2000;191:495-502. [PubMed] [Cited in This Article: ] |
| 18. | Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025-1031. [PubMed] [Cited in This Article: ] |
| 19. | Padigel UM, Kim N, Choi Y, Farrell JP. TRANCE-RANK costimulation is required for IL-12 production and the initiation of a Th1-type response to Leishmania major infection in CD40L-deficient mice. J Immunol. 2003;171:5437-5441. [PubMed] [Cited in This Article: ] |
| 20. | Schimpl A, Berberich I, Kneitz B, Krämer S, Santner-Nanan B, Wagner S, Wolf M, Hünig T. IL-2 and autoimmune disease. Cytokine Growth Factor Rev. 2002;13:369-378. [PubMed] [Cited in This Article: ] |
| 21. | Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, Child JA, Dunstan C, Felsburg PJ, Morgan GJ. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19:849-861. [PubMed] [Cited in This Article: ] |
| 22. | Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260-1268. [PubMed] [Cited in This Article: ] |
| 23. | Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610-615. [PubMed] [Cited in This Article: ] |
| 24. | Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309-319. [PubMed] [Cited in This Article: ] |
| 25. | Stolina M, Dwyer D, Ominsky MS, Corbin T, Van G, Bolon B, Sarosi I, McCabe J, Zack DJ, Kostenuik P. Continuous RANKL inhibition in osteoprotegerin transgenic mice and rats suppresses bone resorption without impairing lymphorganogenesis or functional immune responses. J Immunol. 2007;179:7497-7505. [PubMed] [Cited in This Article: ] |
| 26. | Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531-564. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1881] [Cited by in RCA: 2160] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 28. | Soontrapa K, Honda T, Sakata D, Yao C, Hirata T, Hori S, Matsuoka T, Kita Y, Shimizu T, Kabashima K. Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc Natl Acad Sci USA. 2011;108:6668-6673. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Clegg CH, Rulffes JT, Haugen HS, Hoggatt IH, Aruffo A, Durham SK, Farr AG, Hollenbaugh D. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111-1122. [PubMed] [Cited in This Article: ] |
| 30. | Mehling A, Loser K, Varga G, Metze D, Luger TA, Schwarz T, Grabbe S, Beissert S. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J Exp Med. 2001;194:615-628. [PubMed] [Cited in This Article: ] |
| 31. | Williamson E, Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol. 2002;169:3606-3612. [PubMed] [Cited in This Article: ] |
| 32. | Totsuka T, Kanai T, Nemoto Y, Tomita T, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Akiba H, Okumura K. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol. 2009;182:6079-6087. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1970] [Cited by in RCA: 2251] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 34. | Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Kuroda Y, Matsuo K. Molecular mechanisms of triggering, amplifying and targeting RANK signaling in osteoclasts. World J Orthop. 2012;In press. [Cited in This Article: ] |
| 36. | Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181-193. [PubMed] [Cited in This Article: ] |
| 38. | Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in RCA: 539] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 39. | Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in RCA: 545] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 40. | Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72-80. [PubMed] [Cited in This Article: ] |
| 41. | Finke D. Fate and function of lymphoid tissue inducer cells. Curr Opin Immunol. 2005;17:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, Hoorweg K, Kraal G, Mebius RE. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182:5439-5445. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Sugiyama M, Nakato G, Jinnohara T, Akiba H, Okumura K, Ohno H, Yoshida H. Expression pattern changes and function of RANKL during mouse lymph node microarchitecture development. Int Immunol. 2012;24:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4:353-362. [PubMed] [Cited in This Article: ] |
| 45. | Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015-1024. [PubMed] [Cited in This Article: ] |
| 46. | Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1342] [Cited by in RCA: 1465] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 47. | Taylor RT, Patel SR, Lin E, Butler BR, Lake JG, Newberry RD, Williams IR. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer's patches. J Immunol. 2007;178:5659-5667. [PubMed] [Cited in This Article: ] |
| 48. | Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, Akiba H, Yagita H, Kiyono H, Williams IR. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738-5747. [PubMed] [Cited in This Article: ] |
| 49. | Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301-332. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 50. | Iweala OI, Nagler CR. Immune privilege in the gut: the establishment and maintenance of non-responsiveness to dietary antigens and commensal flora. Immunol Rev. 2006;213:82-100. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833-844. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 52. | Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 53. | Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267-1272. [PubMed] [Cited in This Article: ] |
| 54. | Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423-437. [PubMed] [Cited in This Article: ] |
| 55. | Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438-450. [PubMed] [Cited in This Article: ] |
| 56. | Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139-176. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1120] [Cited by in RCA: 1126] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 57. | Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 58. | Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in RCA: 436] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 59. | Gill J, Malin M, Sutherland J, Gray D, Hollander G, Boyd R. Thymic generation and regeneration. Immunol Rev. 2003;195:28-50. [PubMed] [Cited in This Article: ] |
| 60. | Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 61. | Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571-606. [PubMed] [Cited in This Article: ] |
| 62. | Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287-312. [PubMed] [Cited in This Article: ] |
| 63. | Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395-1401. [PubMed] [Cited in This Article: ] |
| 64. | Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol. 2010;6:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 65. | Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in RCA: 363] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 66. | Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 67. | Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 68. | Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308:248-251. [PubMed] [Cited in This Article: ] |
| 69. | DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727-2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 70. | Giraud M, Taubert R, Vandiedonck C, Ke X, Lévi-Strauss M, Pagani F, Baralle FE, Eymard B, Tranchant C, Gajdos P. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature. 2007;448:934-937. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 72. | Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441:988-991. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 73. | Bleul CC, Corbeaux T, Reuter A, Fisch P, Mönting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 74. | Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature. 2001;414:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in RCA: 605] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 76. | Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331-340. [PubMed] [Cited in This Article: ] |
| 77. | Macleod AS, Havran WL. Functions of skin-resident γδ T cells. Cell Mol Life Sci. 2011;68:2399-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM. Rank signaling links the development of invariant γδ T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 79. | Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 80. | Mouri Y, Yano M, Shinzawa M, Shimo Y, Hirota F, Nishikawa Y, Nii T, Kiyonari H, Abe T, Uehara H. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol. 2011;186:5047-5057. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1181] [Cited by in RCA: 1446] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 82. | Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 83. | Vaira S, Johnson T, Hirbe AC, Alhawagri M, Anwisye I, Sammut B, O'Neal J, Zou W, Weilbaecher KN, Faccio R. RelB is the NF-kappaB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci USA. 2008;105:3897-3902. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 84. | Maruyama T, Fukushima H, Nakao K, Shin M, Yasuda H, Weih F, Doi T, Aoki K, Alles N, Ohya K. Processing of the NF-kappa B2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J Bone Miner Res. 2010;25:1058-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Soysa NS, Alles N, Weih D, Lovas A, Mian AH, Shimokawa H, Yasuda H, Weih F, Jimi E, Ohya K. The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res. 2010;25:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Shinzawa M, Maruyama Y, Qin J, Akiyama N, Miyauchi M, Yanai H, Takami M, Inoue J, Akiyama T. Splenic extramedullary hemopoiesis caused by a dysfunctional mutation in the NF-κB-inducing kinase gene. Biochem Biophys Res Commun. 2011;414:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067-2075. [PubMed] [Cited in This Article: ] |
| 88. | Kinoshita D, Hirota F, Kaisho T, Kasai M, Izumi K, Bando Y, Mouri Y, Matsushima A, Niki S, Han H. Essential role of IkappaB kinase alpha in thymic organogenesis required for the establishment of self-tolerance. J Immunol. 2006;176:3995-4002. [PubMed] [Cited in This Article: ] |
| 89. | Qin J, Konno H, Ohshima D, Yanai H, Motegi H, Shimo Y, Hirota F, Matsumoto M, Takaki S, Inoue J. Developmental stage-dependent collaboration between the TNF receptor-associated factor 6 and lymphotoxin pathways for B cell follicle organization in secondary lymphoid organs. J Immunol. 2007;179:6799-6807. [PubMed] [Cited in This Article: ] |