Copyright
©The Author(s) 2023.
World J Orthop. Apr 18, 2023; 14(4): 218-230
Published online Apr 18, 2023. doi: 10.5312/wjo.v14.i4.218
Published online Apr 18, 2023. doi: 10.5312/wjo.v14.i4.218
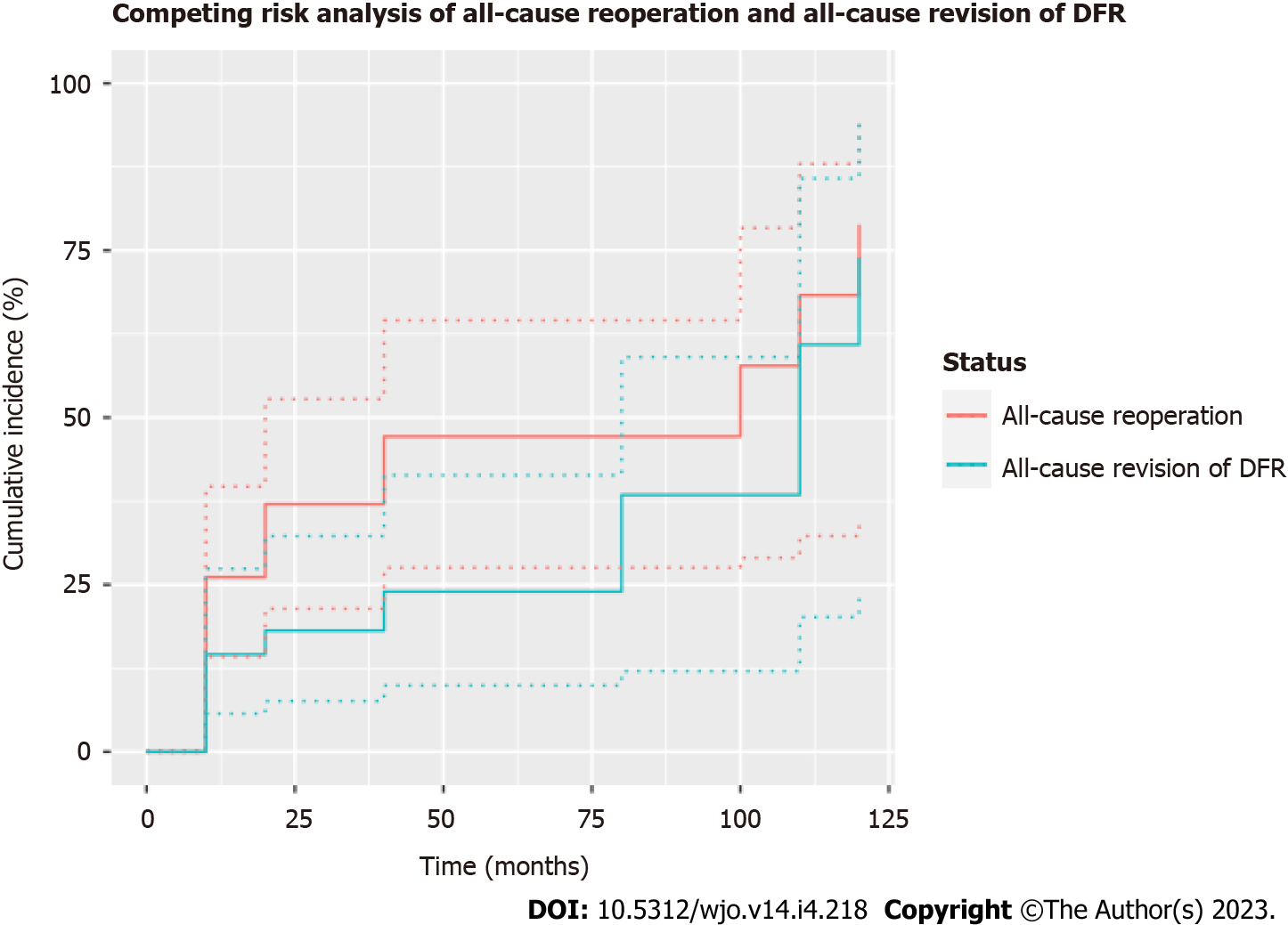
Figure 2 Competing risk analysis for cemented distal femoral replacement with all-polyethylene tibial component constructs for oncologic indications with all-cause revision (femoral or tibial component) and all-cause reoperation as the primary endpoints.
One- and three-year cumulative incidences were 14.6% (95%CI 5.7%-27.4%) and 24.0% (95%CI 9.9%-41.4%), respectively, with all-cause revision as the endpoint. One- and three-year cumulative incidences were 26.1% (95%CI 14.2%-39.7%) and 47.2% (95%CI 27.5%-64.5%), respectively, with all-cause reoperation as the endpoint. DFR: Distal femoral replacement.
- Citation: Christ AB, Chung BC, Urness M, Mayer LW, Gettleman BS, Heckmann ND, Menendez LR. Clinical outcomes of cemented distal femur replacements with all-polyethylene tibial components for oncologic indications. World J Orthop 2023; 14(4): 218-230
- URL: https://www.wjgnet.com/2218-5836/full/v14/i4/218.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i4.218