Copyright
©The Author(s) 2021.
World J Orthop. Mar 18, 2021; 12(3): 119-128
Published online Mar 18, 2021. doi: 10.5312/wjo.v12.i3.119
Published online Mar 18, 2021. doi: 10.5312/wjo.v12.i3.119
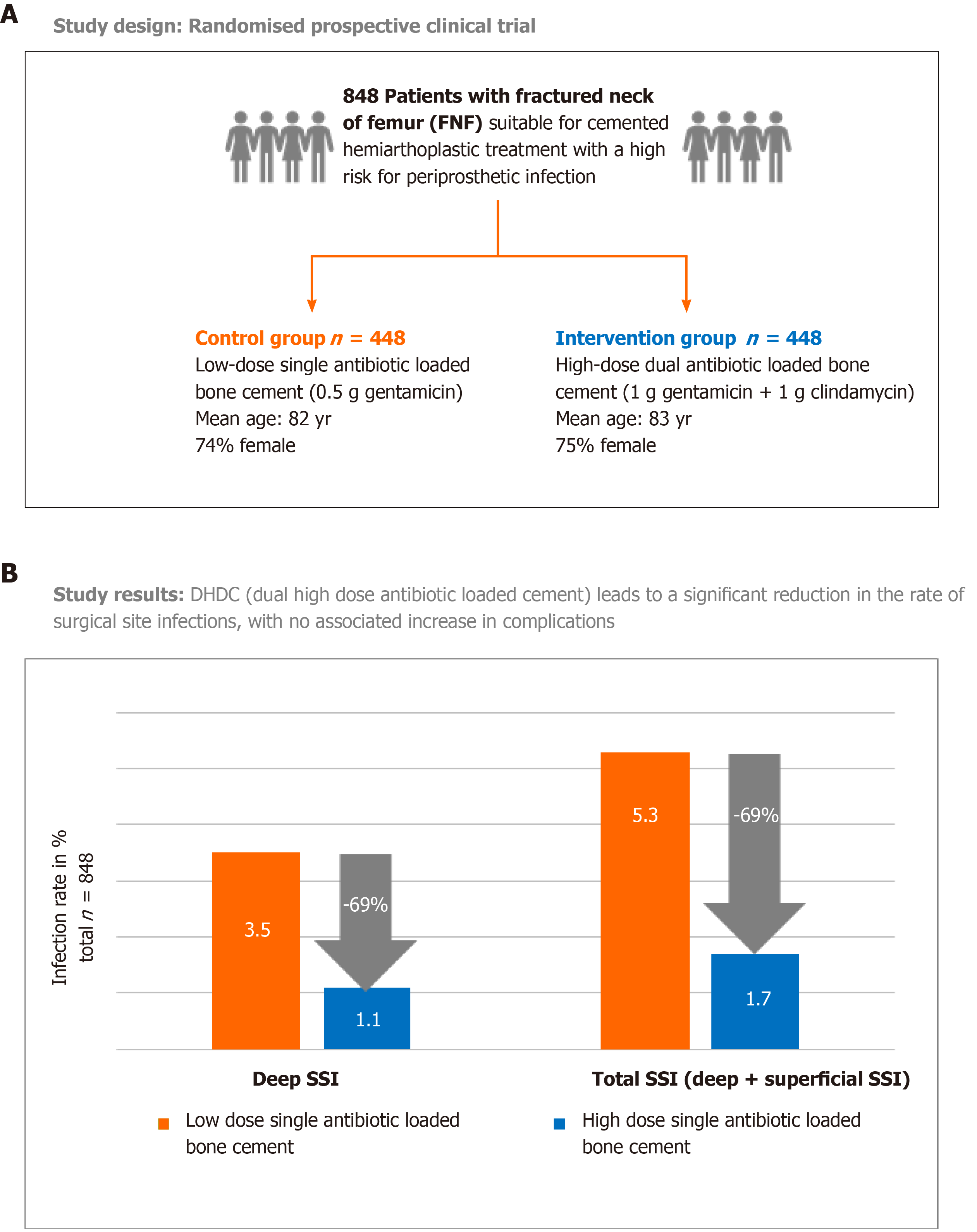
Figure 2 Randomized clinical trial in femoral neck fracture patients comparing prosthetic joint infection rate in low dose single antibiotic loaded bone cement group with high dose dual antibiotic loaded bone cement group.
A: Study design, 848 patients were randomised to receive either hemiprostheses cemented with a low dose single antibiotic-loaded bone cement (PALACOS R + gentamicin = control group) or with a high dose dual antibiotic-loaded bone cement (COPAL gentamicin + clindamycin = intervention group); B: Study results: Primary endpoint was the deep surgical site infection rate (SSI) in the observation period of ≥ 1 yr in each group. Secondary endpoint was the rate of superficial SSI. For the calculation of the total SSI, both deep and superficial SSI cases in each group were combined. SSI: Surgical site infection.
- Citation: Berberich CE, Josse J, Laurent F, Ferry T. Dual antibiotic loaded bone cement in patients at high infection risks in arthroplasty: Rationale of use for prophylaxis and scientific evidence. World J Orthop 2021; 12(3): 119-128
- URL: https://www.wjgnet.com/2218-5836/full/v12/i3/119.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i3.119