Copyright
©The Author(s) 2018.
World J Clin Oncol. Aug 13, 2018; 9(4): 60-70
Published online Aug 13, 2018. doi: 10.5306/wjco.v9.i4.60
Published online Aug 13, 2018. doi: 10.5306/wjco.v9.i4.60
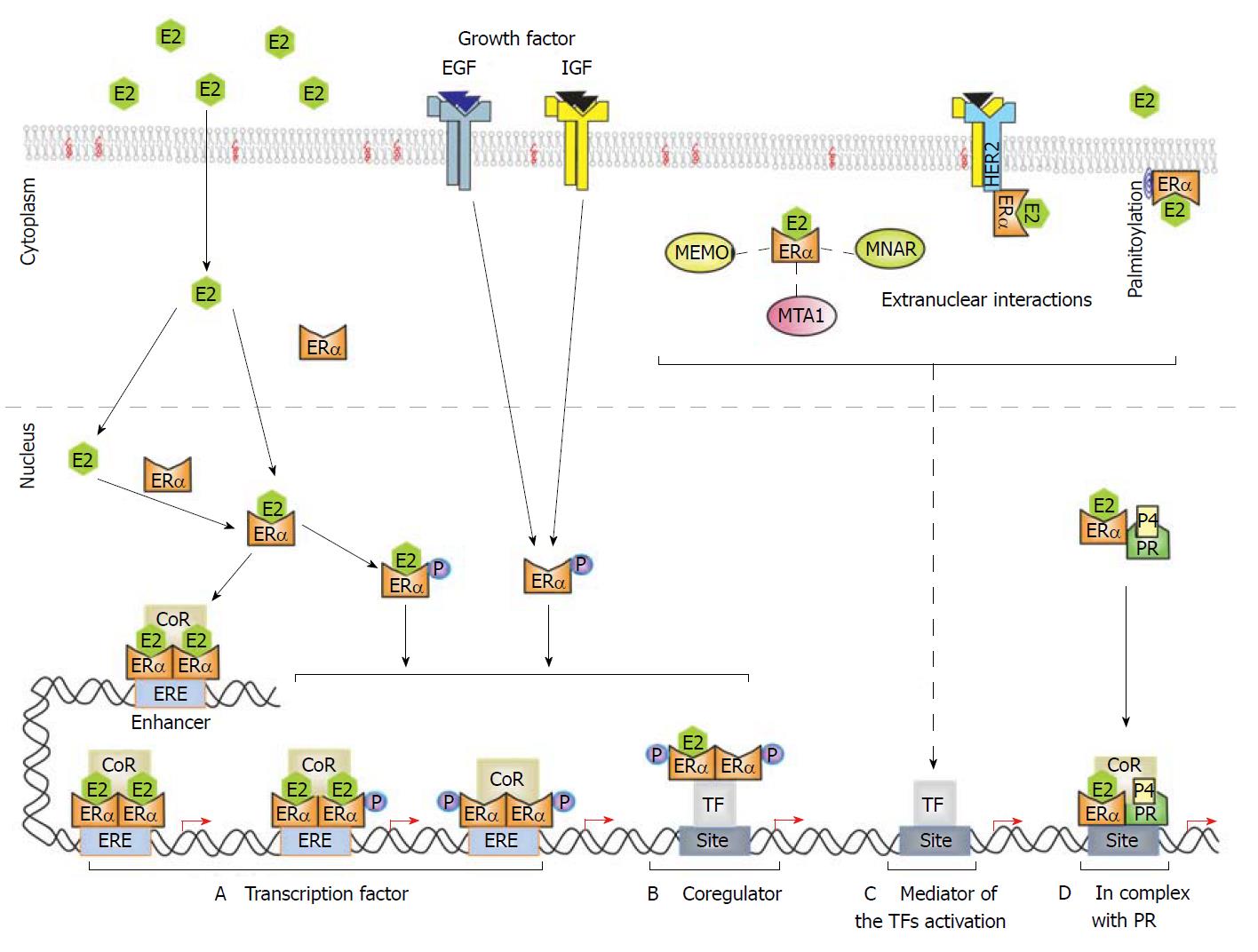
Figure 2 Nuclear and extranuclear signaling of estrogen receptor α.
E2 binds to ERα in the cytoplasm and/or nucleus. Then ERα forms homodimers that recognize the ERE sequence (AGGTCAnnnTGACCT) in target enhancers and promoters, recruiting coregulator (CoR) complexes such as coactivators to induce gene expression. ERα phosphorylation can be induced by E2 to modulate its activity as a transcription regulator. A and B: Growth factors (epidermal growth factor and insulin-like growth factor) also induce ERα phosphorylation in an E2-independent manner to promote ERα activity as a transcription factor or CoR for some transcription factors (i.e., AP-1, Sp1, and NF-κB); C: Cell membrane-associated ERα (via palmitoylation) associated with transmembranal receptors (i.e., HER2) or with cytoplasmatic proteins as (i.e., MEMO, MTA1 and MNAR). These extranuclear interactions can induce kinase–dependent signaling that could finalize in the activation of some transcription factors; D: PR can associate with ERα to coordinate the binding of ERα to the chromatin modulating the expression of specific genes.
- Citation: Tecalco-Cruz AC, Ramírez-Jarquín JO. Polyubiquitination inhibition of estrogen receptor alpha and its implications in breast cancer. World J Clin Oncol 2018; 9(4): 60-70
- URL: https://www.wjgnet.com/2218-4333/full/v9/i4/60.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i4.60