Copyright
©The Author(s) 2018.
World J Clin Oncol. Aug 13, 2018; 9(4): 60-70
Published online Aug 13, 2018. doi: 10.5306/wjco.v9.i4.60
Published online Aug 13, 2018. doi: 10.5306/wjco.v9.i4.60
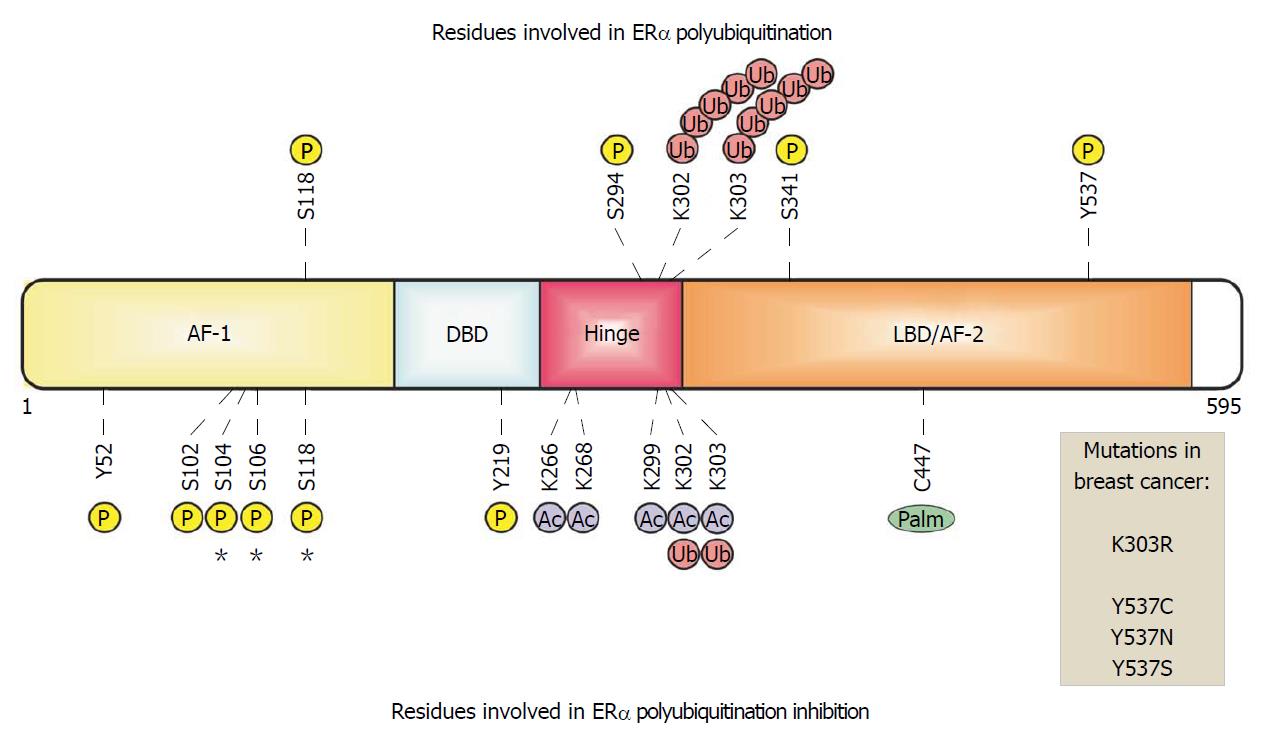
Figure 1 Estrogen receptor α in breast cancer cells.
ERα is organized in functional domains. The transactivation domains AF-1 and AF-2 recruit both coactivators and corepressors. The DNA-binding domain (DBD) recognizes and binds to estrogen response elements in enhancers or promoters. The ligand-binding domain (LBD) is recognized and activated by the 17 beta estradiol hormone. The hinge domain links LBD and DBD allowing the conformational changes of this receptor. Some residues are modified by phosphorylation, acetylation, ubiquitination and palmitoylation , which are related with ERα polyubiquitination. Sites of phosphorylation or mutations in ERα that have been identified in breast–cancer biopsy samples are indicated.
- Citation: Tecalco-Cruz AC, Ramírez-Jarquín JO. Polyubiquitination inhibition of estrogen receptor alpha and its implications in breast cancer. World J Clin Oncol 2018; 9(4): 60-70
- URL: https://www.wjgnet.com/2218-4333/full/v9/i4/60.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i4.60