Published online Aug 10, 2017. doi: 10.5306/wjco.v8.i4.371
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: May 5, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: August 10, 2017
Processing time: 189 Days and 12.5 Hours
Sodium dichloroacetate (DCA) has been studied as a metabolic cancer therapy since 2007, based on a publication from Bonnet et al demonstrating that DCA can induce apoptosis (programmed cell death) in human breast, lung and brain cancer cells. Classically, the response of cancer to a medical therapy in human research is measured by Response Evaluation Criterial for Solid Tumours definitions, which define “response” by the degree of tumour reduction, or tumour disappearance on imaging, however disease stabilization is also a beneficial clinical outcome. It has been shown that DCA can function as a cytostatic agent in vitro and in vivo, without causing apoptosis. A case of a 32-year-old male is presented in which DCA therapy, with no concurrent conventional therapy, resulted in regression and stabilization of recurrent metastatic melanoma for over 4 years’ duration, with trivial side effects. This case demonstrates that DCA can be used to reduce disease volume and maintain long-term stability in patients with advanced melanoma.
Core tip: Sodium dichloroacetate (DCA) has been studied as a metabolic cancer therapy since 2007. It has been shown that DCA therapy can result in a classic response which is measured by reduction or disappearance of tumours on imaging. However, DCA can also halt cancer cell growth without causing apoptosis (cytostatic effect). This can result in long-term stabilization of metastatic cancer. We present a case of oral DCA therapy resulting in reduction and stabilization of metastatic melanoma in a 32-year-old male for over 4 years, with only minor side effects.
- Citation: Khan A, Andrews D, Shainhouse J, Blackburn AC. Long-term stabilization of metastatic melanoma with sodium dichloroacetate. World J Clin Oncol 2017; 8(4): 371-377
- URL: https://www.wjgnet.com/2218-4333/full/v8/i4/371.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i4.371
Sodium dichloroacetate (DCA) caught the attention of the medical community in 2007, when Bonnet et al[1] published the first in vitro and in vivo study illustrating the value of DCA as a metabolic cancer therapy, through its inhibitory action on the mitochondrial enzyme pyruvate dehydrogenase kinase. Previously, Stacpoole et al[2-4] had published several studies of DCA for the treatment of congenital lactic acidosis in mitochondrial diseases[2-5]. These studies demonstrated that oral DCA is a safe drug for human use. DCA was noted to have an absence of renal, pulmonary, bone marrow and cardiac toxicity[4]. Most DCA side effects were modest, with the most serious one being reversible peripheral neuropathy[6]. Reversible delirium has also been reported[7]. Elevation of liver enzymes (asymptomatic and reversible) has been noted in a small percentage of patients[3]. The prior human research in mitochondrial disorders has enabled the rapid translation of DCA into human use as an off-label cancer therapy. Several reports of clinical trials using DCA as cancer therapy have now been published, confirming its safety profile, and indicating an increasing recognition of the potential usefulness of DCA in the cancer clinic[8-11]. One limitation of these studies involving late stage patients is that they have only reported on treatment for short periods of time.
In Bonnet’s 2007 publication[1], DCA treatment was shown to reduce mitochondrial membrane potential which promoted apoptosis selectively in human cancer cells. Aerobic glycolysis inhibition (the Warburg effect) and mitochondrial potassium ion channel activation were identified as the mechanisms of action of DCA. Further investigations of DCA in vitro have confirmed the anti-cancer activity against a wide range of cancer types, which have been reviewed recently by Kankotia and Stacpoole[12]. In addition, DCA is also able to enhance apoptosis when combined with other agents[13-15]. Other anticancer actions of DCA have also been suggested, including angiogenesis inhibition[16], alteration of HIF1-α expression[17], alteration of cell pH regulators V-ATPase and MCT1, and other cell survival regulators such as p53 and PUMA[18]. However, many in vitro studies use unreasonably high concentrations of DCA that are not clinically achievable, in an effort to show cytotoxic activity[12]. In other studies, more modest DCA concentrations were used, demonstrating that DCA could be cytostatic. The second report in 2010 of its in vivo anti-cancer activity found DCA alone to be cytostatic in a metastatic model of breast cancer[19], inhibiting proliferation without triggering apoptosis. This suggests a role for DCA as a cancer stabilizer, similar to angiogenesis inhibitors.
In response to the 2007 report of the anti-cancer actions of DCA, Khan began using DCA for the treatment of cancer patients with short prognosis or who had stopped responding to conventional cancer therapies. A natural medication protocol was developed in collaboration with a naturopathic physician (Andrews) to address the dose-limiting neurologic toxicity of DCA. This consisted of 3 medicines: Acetyl L-carnitine[20-22], R-alpha lipoic acid[23-25] and benfotiamine[26-28], for neuropathy and encephalopathy prevention. In over 300 advanced stage cancer patients, observational data revealed that DCA therapy benefitted 60%-70% of cases. The neuropathy risk when natural neuroprotective medicines were combined with DCA was approximately 20% using 20-25 mg/kg per day dosing on a 2 wk on/1 wk off cycle (clinic observational data published online at http://www.medicorcancer.com). Here, a patient case report illustrating both the apoptotic and anti-proliferative effects of chronic DCA treatment over a period of over four years is presented.
A 32 years old previously healthy fair-skinned male originally noted that a mole on his left calf began to change in 2006. He consulted a doctor and the mole was excised. A pathologic diagnosis of melanoma was made. A sentinel node dissection was carried out, and was negative for metastatic disease. In 2007, the patient noted enlargement of left inguinal lymph nodes, and small melanocytic lesions on the skin of his left leg. He was treated with interferon alpha under a clinical trial at a regional cancer hospital, with reduction of the nodes and resolution of the skin metastases. Interferon was stopped after 9 mo due to side effects.
The patient remained well until 2010, when a new left leg skin metastasis appeared. This was surgically excised. In late 2011, another new cutaneous metastasis was identified on the left leg, within the scar from the original melanoma surgery. This was biopsied and a diagnosis of recurrent melanoma was confirmed. He was then treated with wide excision and skin graft.
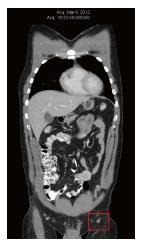
In March 2012, the patient was diagnosed with a recurrence within the left leg skin graft. This was excised and a new skin graft procedure was performed. Pathology revealed positive margins of the excised metastasis, so a re-excision was performed, again with positive margins. At the same time, needle biopsy of a left inguinal lymph node confirmed the presence of BRAF-positive metastatic melanoma. A Computed tomography (CT) scan performed in Mar 2012 revealed no evidence of distant metastases. The largest left inguinal node was 8mm in diameter, which was reported as “insignificant by size criteria” (Figure 1).
In April 2012, the patient consulted a naturopathic doctor (Shainhouse) and began therapy with the following oral natural anti-cancer agents: Active hexose correlated compound or AHCC (mushroom extract)[29], dandelion root[30], curcumin[31], and astragalus root[32]. Parenteral therapy was also started, which consisted of intravenous vitamin C twice weekly[33] and subcutaneous European mistletoe extract[34]. The patient also changed to a vegan diet.
In May 2012, the patient attended the author’s clinic (Khan) looking to pursue additional non-traditional therapies. DCA therapy was discussed, but the patient decided to give the natural anti-cancer therapies (prescribed by Shainhouse) an adequate trial first. CT scan was performed again in May 2012 (after only 1 mo of natural therapy) and indicated mild growth of multiple inguinal and external iliac nodes, with sizes ranging from 10 mm × 11 mm to 14 mm × 15 mm.
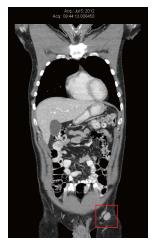
In July 2012, CT scan was repeated to assess the patient’s natural anti-cancer therapies. At that time, the left inguinal and external iliac nodes had enlarged again, and ranged in size from 13 mm × 16 mm to 22 mm × 20 mm (Figure 2). PET scan was also performed in preparation for entering a clinical trial in Boston, MA (United States), and confirmed increased glucose uptake in the left inguinal nodes. There was new low intensity (2/10) aching pain in the left inguinal region. Examination revealed a 20 mm non-tender left inguinal lymph node, and two small skin metastases within the left calf skin graft.
The patient was thus diagnosed with disease progression. At that point he decided to initiate DCA therapy. He began oral DCA 500 mg 3 times per day, which was equivalent to 17 mg/kg per day (manufacturer: Tokyo Chemical Industry, United States) in addition to maintaining the other natural therapies. The DCA treatment cycle was 2 wk on and 1 wk off. To minimize the occurrence of DCA side effects, 3 additional natural medications were prescribed: Oral acetyl L-carnitine 500 mg 3 times a day, oral benfotiamine 80 mg twice a day and oral R-alpha lipoic acid 150 mg 3 times a day. These supplements were taken daily (no cycle). Routine baseline blood tests were performed (Table 1). These were all normal, except for low creatinine which was felt to be insignificant.
| Blood test | July 12 pre-DCA | October 12 3 mo DCA | June 16 4 yr DCA | Units | Normal range |
| Hemoglobin | 154 | 150 | 157 | g/L | 135-175 |
| White cell count | 4.5 | 4.1 | 5 | × 109/L | 4.0-11.0 |
| Platelets | 220 | 214 | 229 | × 109/L | 150-400 |
| Glucose | - | 4.6 | 4.9 | mmol/L | 3.6-7.7 |
| Urea | 3.9 | 3.2 | 3.9 | mmol/L | 2.5-8.0 |
| Creatinine | 491 | 501 | 551 | µmol/L | 62-115 |
| Calcium | 2.47 | 2.41 | 2.47 | mmol/L | 2.15-2.60 |
| Albumin | 48 | 45 | 47 | g/L | 35-50 |
| Bilirubin | 8 | 10 | 13 | µmol/L | < 22 |
| Sodium | 139 | 141 | 140 | mmol/L | 135-147 |
| Potassium | 4 | 4.3 | 3.9 | mmol/L | 3.5-5.5 |
| Chloride | 106 | 107 | 105 | mmol/L | 100-110 |
| Alkaline Phosphatase | 77 | 69 | 71 | U/L | 45-129 |
| LDH | 139 | 135 | 144 | U/L | 120-246 |
| GGT | 18 | 19 | 20 | U/L | 15-73 |
| AST | 18 | 25 | 21 | U/L | 7-37 |
| ALT | 18 | 28 | 19 | U/L | 12-49 |
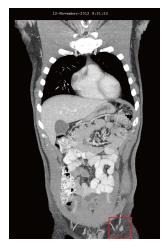
In November 2012, 4 mo after the addition of DCA to his original natural anti-cancer therapies, the patient was re-assessed. He felt generally well. Two new symptoms were reported to have begun only after initiation of DCA therapy: Slightly reduced sensation of the finger tips and toes, and slightly reduced ability to concentrate during the 2 wk periods in which he was taking DCA. The mild sensory loss was not worsening and was felt to be mild DCA-related neuropathy. Both the numbness and reduced concentration were reported to resolve during the weeks when the patient was off DCA. Blood panel from October 2012 showed no significant changes (Table 1). August 2012 and November 2012 CT scans revealed significant regression of all previously enlarged lymph nodes. The largest node was 10 mm, and there was no evidence of intra-thoracic or intra-abdominal disease, and no bone metastases (Figure 3).
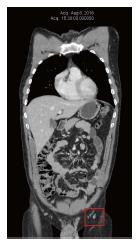
The patient continued to feel well on DCA therapy, and did not notice any new skin metastases or new enlargement of inguinal nodes. He continued to have frequent clinical monitoring with his naturopathic doctor (Shainhouse), and annual follow-up with his medical doctor (Khan). The listed natural anti-cancer therapies (prescribed by Shainhouse) and DCA therapy were maintained into 2016. Blood panel results in June 2016 continued to be normal (Table 1). CT scan was repeated in August 2016, showing no evidence of metastatic melanoma, after a full 4 years of ongoing DCA therapy, combined with natural anti-cancer therapy (Figure 4). By December 2016, the patient reported an increase in work-related stress and a reduction in compliance with his medications. At the time, he noted a new left inguinal mass. Ultrasound imaging was obtained, which revealed a new conglomerate of enlarged lymph nodes measuring 40 mm × 25 mm × 23 mm, with colour Doppler showing blood flow within the mass. This was interpreted as re-growth of melanoma, after approximately four and a half years of continuous DCA therapy. Further workup was performed including a PET/CT scan, which confirmed disease recurrence in 3 left inguinal nodes (SUVmax ranging from 13 to 17.8).
In summary, the patient received conventional therapy for recurrent stage 3 melanoma over a period of 6 years, consisting of primary surgical excision with lymph node dissection, interferon alpha and surgical excisions for recurrent cutaneous metastases on 5 occasions. The patient then received natural anti-cancer therapy alone (prescribed by Shainhouse) for 3 mo with no response, evidenced by steady disease progression on serial CT scans. Finally the patient added oral DCA therapy to the natural anti-cancer therapy, with 3 concurrent neuroprotective medicines (lipoic acid, acetyl L-carnitine and benfotiamine) and no concurrent conventional cancer therapies. The result was a complete radiological remission lasting for over 4 years, followed by recurrence. During the course of DCA therapy, the patient experienced trivial side effects consisting of slight neuropathy and slight reduction of concentration. The patient maintained ECOG level 0 function, and he was able to work full time.
The use of oral DCA in the metastatic melanoma patient described herein demonstrates tumour shrinkage and long-term disease stability according to clinical status and CT imaging. Disease stability was maintained for over 4 years while taking DCA in the absence of any concurrent conventional therapy, with a survival time since the initial diagnosis of 10 years. According to the National Cancer Institute’s SEER cancer statistics, the survival of this patient who showed no evidence of distant metastases is not remarkable (62.9% 5-year survival rate for melanoma with spread to regional lymph nodes, https://seer.cancer.gov/statfacts/html/melan.html). What is remarkable is that in a situation where involved lymph nodes were clearly enlarging, the addition of oral DCA therapy was efficacious in shrinking the enlarging nodes (Figures 2 and 3), and in achieving a remission lasting over 4 years. It is possible that the natural anti-cancer therapies the patient received synergized with DCA, but it is also clear that these natural therapies alone cannot account for the disease regression. DCA has been reported to have both apoptotic and cytostatic effects[14,17,19,35,36], which is consistent with this patient’s clinical course of regression (apoptotic) and prolonged remission (cytostatic). The recurrence after 4 years coincided with reduced compliance, suggesting that this method of cancer management with DCA requires the metabolic pressure to be maintained continuously. Despite recurrence, the patient remained clinically well and planned to start new immunotherapy medications. It remains to be seen if a change in therapy can once again achieve disease regression or stability.
In addition to the maintenance of remission for over 4 years, this case illustrates that DCA can be well-tolerated in a cancer patient for a prolonged time period, as compared to all published DCA cancer clinical trials. Notably, this patient was able to tolerate 17 mg/kg per day in a regime of 2 wk on/1 wk off for 4 years with minimal side effects. This is similar to our previous case report of chronic DCA usage in colon cancer[37], where the patient was able to tolerate 16 mg/kg per day (but not 25 mg/kg per day) in the same regime, but contrasts with the clinical trials for DCA, which recommend a lower dose of 10-12.5 mg/kg per day given continuously[9,11]. The 1 wk break or the neuroprotective supplements may both contribute to the ability of the patients in the case reports to tolerate the higher dose. Genetic polymorphisms in GSTZ1, the liver enzyme that metabolises DCA, may also contribute to the dose of DCA that can be tolerated[9,38]. Variable drug levels have been reported in the trials, but not all of them have considered this pharmacogenetic aspect of DCA therapy[9,11], and further studies are needed to clarify if this is a significant contributor to DCA tolerance. As of this writing, a DCA multiple myeloma human trial is ongoing, which is examining both GSTZ1 genotypes and drug levels to contribute to our understanding of these issues (Australia New Zealand Clinical Trials Register #ACTRN12615000226505, http://www.anzctr.org.au).
This case report shows that chronic DCA therapy can be used without reducing quality of life, as compared to conventional melanoma therapies such as interferon. To determine the optimal protocol for maximum tolerable acute or chronic treatment with DCA, human trials are needed. But more importantly, it still remains to be clarified what dose is required for on-target effects that will be efficacious against cancer. This information is necessary before investing in larger, long term studies on patient outcomes. DCA deserves further investigation in clinical trials as a non-toxic cancer therapy due to its modest cost and low toxicity, and deserves consideration as an off-label cancer therapy.
The authors wish to thank Dr. Humaira Khan for her assistance, and also the patient for his support and consent to publish his case.
The 32-year-old male patient presented with a pigmented lesion on his leg.
The patient was diagnosed with a melanoma.
Melanoma confirmed by excisional biopsy.
Enlarged inguinal node confirmed to be involved with melanoma (needle biopsy).
Melanoma, BRAF positive.
Excision of primary lesion with skin graft, sentinel node dissection, multiple excisions of recurrent cutaneous metastases. Traditional therapy stopped and natural anti-cancer therapies started (AHCC, dandelion root, curcumin, astragalus root, i.v. vitamin C, s.c. European mistletoe). Progression after 3 mo, dichloroacetate (DCA) added. Regression and remission following addition of DCA lasting for over 4 years.
Computed tomography scan reports demonstrate the course of the disease and response to therapies.
DCA: Dichloroacetate sodium; RECIST: Response Evaluation Criteria for Solid Tumours; ECOG: Eastern Cooperative Oncology Group.
DCA can act as a pro-apoptotic and cytostatic drug, and can thus achieve regression as well as long-term stabilization of metastatic cancer without serious side effects, as illustrated by this melanoma case.
Dr. Khan described a 32-year-old man received DCA therapy, with other medications from natural therapists and maintained in a stabilization state (metastatic melanoma) for over 4 years. It is an interesting case.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Peters GJ, Su CC S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1156] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 2. | Stacpoole PW, Kurtz TL, Han Z, Langaee T. Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev. 2008;60:1478-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, Shuster JJ. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223-e1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Berendzen K, Theriaque DW, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6:126-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Brandsma D, Dorlo TP, Haanen JH, Beijnen JH, Boogerd W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. J Neurol. 2010;257:2099-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 9. | Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014;32:452-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar F. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2014;140:443-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33:603-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Kankotia S, Stacpoole PW. Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta. 2014;1846:617-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Sun RC, Board PG, Blackburn AC. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol Cancer. 2011;10:142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Stockwin LH, Yu SX, Borgel S, Hancock C, Wolfe TL, Phillips LR, Hollingshead MG, Newton DL. Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. Int J Cancer. 2010;127:2510-2519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Gang BP, Dilda PJ, Hogg PJ, Blackburn AC. Targeting of two aspects of metabolism in breast cancer treatment. Cancer Biol Ther. 2014;15:1533-1541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Sutendra G, Dromparis P, Kinnaird A, Stenson TH, Haromy A, Parker JM, McMurtry MS, Michelakis ED. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2013;32:1638-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Cairns RA, Bennewith KL, Graves EE, Giaccia AJ, Chang DT, Denko NC. Pharmacologically increased tumor hypoxia can be measured by 18F-Fluoroazomycin arabinoside positron emission tomography and enhances tumor response to hypoxic cytotoxin PR-104. Clin Cancer Res. 2009;15:7170-7174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Anderson KM, Jajeh J, Guinan P, Rubenstein M. In vitro effects of dichloroacetate and CO2 on hypoxic HeLa cells. Anticancer Res. 2009;29:4579-4588. [PubMed] [Cited in This Article: ] |
| 19. | Sun RC, Fadia M, Dahlstrom JE, Parish CR, Board PG, Blackburn AC. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat. 2010;120:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 183] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 20. | De Grandis D. Acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: a short review. CNS Drugs. 2007;21 Suppl 1:39-43; discussion 45-46. [PubMed] [Cited in This Article: ] |
| 21. | Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crinò L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005;91:135-138. [PubMed] [Cited in This Article: ] |
| 22. | Evans JD, Jacobs TF, Evans EW. Role of acetyl-L-carnitine in the treatment of diabetic peripheral neuropathy. Ann Pharmacother. 2008;42:1686-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Liu F, Zhang Y, Yang M, Liu B, Shen YD, Jia WP, Xiang KS. [Curative effect of alpha-lipoic acid on peripheral neuropathy in type 2 diabetes: a clinical study]. Zhonghua Yixue Zazhi. 2007;87:2706-2709. [PubMed] [Cited in This Article: ] |
| 25. | Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schütte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia. 1995;38:1425-1433. [PubMed] [Cited in This Article: ] |
| 26. | Winkler G, Kempler P. [Pathomechanism of diabetic neuropathy: background of the pathogenesis-oriented therapy]. Orv Hetil. 2010;151:971-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Ang CD, Alviar MJ, Dans AL, Bautista-Velez GG, Villaruz-Sulit MV, Tan JJ, Co HU, Bautista MR, Roxas AA. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;CD004573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Winkler G, Pál B, Nagybéganyi E, Ory I, Porochnavec M, Kempler P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung. 1999;49:220-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Ignacio RM, Kim CS, Kim YD, Lee HM, Qi XF, Kim SK. Therapeutic effect of Active Hexose-Correlated Compound (AHCC) combined with CpG-ODN (oligodeoxynucleotide) in B16 melanoma murine model. Cytokine. 2015;76:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Chatterjee SJ, Ovadje P, Mousa M, Hamm C, Pandey S. The efficacy of dandelion root extract in inducing apoptosis in drug-resistant human melanoma cells. Evid Based Complement Alternat Med. 2011;2011:129045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Mirzaei H, Naseri G, Rezaee R, Mohammadi M, Banikazemi Z, Mirzaei HR, Salehi H, Peyvandi M, Pawelek JM, Sahebkar A. Curcumin: A new candidate for melanoma therapy? Int J Cancer. 2016;139:1683-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 32. | Huang XY, Zhang SZ, Wang WX. Enhanced antitumor efficacy with combined administration of astragalus and pterostilbene for melanoma. Asian Pac J Cancer Prev. 2014;15:1163-1169. [PubMed] [Cited in This Article: ] |
| 33. | Wagner SC, Markosian B, Ajili N, Dolan BR, Kim AJ, Alexandrescu DT, Dasanu CA, Minev B, Koropatnick J, Marincola FM. Intravenous ascorbic acid as an adjuvant to interleukin-2 immunotherapy. J Transl Med. 2014;12:127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;CD003297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Delaney LM, Ho N, Morrison J, Farias NR, Mosser DD, Coomber BL. Dichloroacetate affects proliferation but not survival of human colorectal cancer cells. Apoptosis. 2015;20:63-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Abildgaard C, Dahl C, Basse AL, Ma T, Guldberg P. Bioenergetic modulation with dichloroacetate reduces the growth of melanoma cells and potentiates their response to BRAFV600E inhibition. J Transl Med. 2014;12:247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Khan A, Andrews D, Blackburn AC. Long-term stabilization of stage 4 colon cancer using sodium dichloroacetate therapy. World J Clin Cases. 2016;4:336-343. [PubMed] [Cited in This Article: ] |
| 38. | Tzeng HF, Blackburn AC, Board PG, Anders MW. Polymorphism- and species-dependent inactivation of glutathione transferase zeta by dichloroacetate. Chem Res Toxicol. 2000;13:231-236. [PubMed] [Cited in This Article: ] |