Published online Nov 10, 2012. doi: 10.5306/wjco.v3.i11.142
Revised: October 22, 2012
Accepted: November 2, 2012
Published online: November 10, 2012
Processing time: 217 Days and 18.5 Hours
AIM: To assess whether the addition of a customized, active immunotherapy to standard of care including fluorescence-guided surgery, may provide hints of an improved survival for patients with poor-prognosis, incurable glioblastoma multiform.
METHODS: Preliminary to our ongoing, phase-II clinical trial, we conducted a small pilot study enrolling five consecutive patients with resectable glioblastoma. In terms of Recursive Partitioning Analysis, four patients were class V and one was class IV. In all five cases, fluorescence-guided surgery was employed, followed by rapid steroid discontinuation. Patients were then treated with a combination of standard radio-chemotherapy with temozolomide and tumor lysate-pulsed, mature dendritic cell-based vaccinations.
RESULTS: Though all five patients ultimately progressed, with any further treatment left to the sole decision of the treating oncologist, active immunotherapy was very well tolerated and induced specific immune responses in all three patients for whom enough material was available for such an assessment. Median progression-free survival was 16.1 mo. Even more important, median and mean overall survival were 27 mo and 26 mo, respectively. Three patients have died with an overall survival of 9 mo, 27 mo and 27.4 mo, while the other two are still alive at 32 mo and 36 mo, the former receiving treatment with bevacizumab, while the latter has now been off therapy for 12 mo. Four of five patients were alive at two years.
CONCLUSION: Active immunotherapy with tumor lysate-pulsed, autologous dendritic cells is feasible, safe, well tolerated and biologically efficacious. A phase-II study is ongoing to possibly improve further on our very encouraging clinical results.
- Citation: Valle RD, Cerio ALD, Inoges S, Tejada S, Pastor F, Villanueva H, Gallego J, Espinos J, Aristu J, Idoate MA, Andreu E, Bendandi M. Dendritic cell vaccination in glioblastoma after fluorescence-guided resection. World J Clin Oncol 2012; 3(11): 142-149
- URL: https://www.wjgnet.com/2218-4333/full/v3/i11/142.htm
- DOI: https://dx.doi.org/10.5306/wjco.v3.i11.142
The prognosis of glioblastoma multiforme (GBM) remains dismal[1]. Radio-chemotherapy’s benefit has been recently proven (14.6 vs 12.1 mo; P < 0.0001), but overall survival (OS) is still short, only 26% of the patients surviving two years[2]. Targeted therapies have added little benefit, with best results in phase II trials reaching a median OS of 19.6 mo and 2 year OS of 37%[3]. Recursive partition analysis had shown that a priori prognostic factors and kind of surgery divide the patients in three classes (III, IV and V) with different prognosis[4], and the benefit of modern therapies concentrates in the better. Present standard therapy showed marginal benefit for class V patients (10.7 mo vs 9.1 mo) in an European Organization for Research and Treatment of Cancer (EORTC)-National Cancer Institute of Canada (NCIC) trial[2].
Several immunotherapy strategies have been attempted and shown to be safe and tolerable. The results of published phase-I/II trials have hinted at efficacy, but they have featured selection criteria and included patient populations, which make comparisons difficult[5-7].
In 2009, we started a phase-II trial for patients with newly-diagnosed GBM based on immunotherapy with ex-vivo tumor lysate-pulsed, autologous dendritic cells (DC) following fluorescence-guided surgery (FGS) using 5-aminolevulinic. After resection, immunotherapy was used as up-front therapy in combination with standard therapy. We hypothesized that the more extensive resection possible with this surgical technique[8,9] would create the best situation to begin immunotherapy, and that immunotherapy itself could be more useful as a front-line strategy. To avoid the potential selection biases shown in other immunotherapy trials[5-7], we aimed at including a wide population of patients and enrolling them right after surgery. Main limitation for entry is that FGS surgery must achieve residual tumor less than 1 cc.
In this report, we present 5 previous cases that constitute a pilot group of this clinical trial.
This pilot study was approved by the institutional review board and the ethical committee of Navarra and all the Spanish regulations concerning human trials were observed.
We screened for entry every patient candidate for resection surgery and five consecutive patients with GBM were enrolled in this pilot study, approved by our institutional review board and abiding the norms of the Declaration of Helsinki. We consider most GBM cases candidates for resection surgery, excluding only patients with bilateral extension through the corpus callosum, multiple distant lesions, or ill-defined mass in eloquent areas.
FGS was done with a Zeiss© Pentero microscope after Gliolan© administration as previously published[8]. Tumor dissection was carried out along the tumor border defined by fluorescence whenever possible[9]. As much tumor tissue as possible was collected, no ultrasonic aspirator was ever used. FGS allows identifying central viable and invasive portions of the tumor with great predictive positive value, as we have shown previously[10]. After surgery, steroids were quickly tapered and discontinued.
Patients underwent standard magnetic resonance imaging (MRI) in a Siemens Symphony 1.5 T or Trio 3 T unit both preoperatively and 48 h after surgery. Preoperative tumor volume and any gadolinium enhancing remnants were measured using Brainlab planning software after manual segmentation.
All samples were evaluated by the same expert neuropathologist and diagnosed as GBM according to the World Health Organization criteria[11]. O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status was assessed by polymerase chain reaction.
All manipulations necessary to produce the vaccines were carried out under good manufacturing practice conditions.
After receiving the tumor sample, a single cell suspension was produced by mechanical disaggregation using the GentleMACS™ Dissociator (Miltenyi Biotec). The cell suspension was immediately frozen and stored until use. To prepare the tumor cells lysate, tumor cells were thawed and subjected to four cycles of freezing and thawing. After centrifugation, the supernatant was filtered with a 40 μm Falcon filter (BD Biosciences) and the amount of proteins obtained was quantified using the BCA™ Protein Assay Kit (Pierce). After irradiation (54 Gy), the tumor lysate was frozen. Central and invasive portions of the tumor were processed separately.
DC were generated from CD14+ monocytes. Peripheral blood mononuclear cells (PBMC) were collected from patients through leukapheresis. CD14+ cells were then selected by immunomagnetic separation using a CliniMACS™ (Miltenyi Biotec) and cultured at 2 × 106 cells/mL in AIM-V (Gibco) supplemented with antibiotics, 1000 UI/ml of interleukin-4 (IL-4) (R and D Systems) and 1000 UI/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leukine™, Genzyme Corporation,) in culture bags (CellGenix). IL-4 (500 UI/mL) and GM-CSF (500 UI/mL) were further added on the fourth day, and cultured cells were harvested on the seventh day. These immature DC were adjusted at 107 cells/mL and pulsed with autologous tumor lysate during 2 h at 37 °C and 5% CO2. At that time, to induce DC maturation, 50 ng/mL of tumor necrosis factor (TNF)-α (Beromun™, Boehringer Ingelheim), 1000 UI/mL of interferon (IFN)-α (Intron A™, Schering Corporation) and 20 ng/mL of Poly I:C (Amersham) were added to the medium, and cells were placed in culture bags at 2 × 106 cells/mL. Mature DC were harvested on the eighth day and frozen in aliquots until use.
Surface markers of both immature and mature DC were ascertained by flow cytometry using a FACSCalibur™ (Becton-Dickinson), whereas data were analyzed using the Cell Quest Pro software (Becton-Dickinson). For each dose of vaccine, one aliquot of frozen DC was thawed. Ten million cells per vaccination were considered the optimal dose, though fewer cells were actually employed in some instances, as specified in the results section. To address the issue of quality assurance, an aliquot of the final product underwent sterility controls.
All patients started radio-chemotherapy following Stupp protocol[2]. The intention was to administer 12 cycles if well tolerated.
The leukapheresis was carried out usually within two weeks from surgery, at least seven days after last dexamethasone dose. First DC administration was scheduled prior to radiotherapy, second one, three weeks after radiotherapy, followed by two monthly, four by-monthly, and later quarterly until the end of all available doses. The actual number of doses received by each patient is specified in the results section. During temozolomide treatment, DC were administered on day 21 of the cycle to benefit from the recovery form leukopenia.
Patients were followed clinically each month during the first year and every 2 mo thereafter.
MRI was carried out at 3 mo intervals or within a week from any neurological worsening. A neuroradiologist evaluated the MRI using the Response Assessment in Neurooncology criteria criteria[12]. When progression was observed, any subsequent therapy was left at the discretion of the treating oncologist. All patients were followed until death.
OS of the patients in this pilot study was compared to that of patients undergoing standard therapy with the published nomograms from EORTC 26981/22981 NCIC trial[13] and to a matched historic cohort. The historic cohort was selected from patients operated in our center with FGS, and similar age, Karnofsky performance status (KPS), recursive partitioning analysis (RPA), MGMT methylation status and residual tumor volume (four complete resections, one less than 1cc). From the nomograms, each patient was assigned an expected OS; if our treated population survival had been similar to the EORTC series, the proportion exceeding median OS should have been 0.5. Binomial test with proportion 0.5 was used to assess statistical relevance. The OS of the treated patients was compared also to historic controls using log-rank (Mantel-Cox).
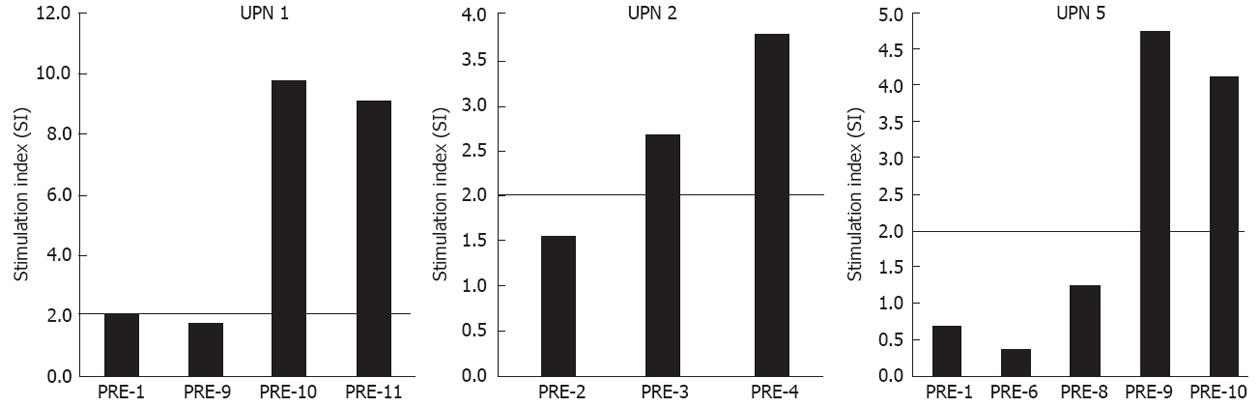
Tumor-specific cellular immune responses were assessed by three independent, controlled methods: T-cell proliferation assay, cytokine release enzyme-linked immunosorbent assay (ELISA) and interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay as previously published[13]. For the T-cell proliferation assays, PBMC were stimulated with 20.000 DC pulsed and matured. The stimulation index (SI) was calculated as the ratio of the response to tumor lysate or pulsed DC over the mean response in the absence of tumor material. A response was considered positive when the SI was > 2 at least at two different time points. IFN-γ and TNF-α production was measured by ELISA (Pharmingen). A response was considered positive when IFN-γ and TNF-α production was > 2 than in controls at least at two different time points.
The number of IFN-γ producing cells was measured by ELISPOT (Mabtech). PBMC were stimulated with 20.000 DC pulsed with tumor lysate and matured. A response was considered positive when the number of spots was > 2 than in controls at least at two different time points.
Five patients were screened and included (Table 1). Mean age was 66 years, median KPS was 70, four patients were RPA class V and one was class IV. MGMT promoter was unmethylated in one and methylated in four cases. Mean preoperative tumor volume was 54 cc (34-112) Resection was total in three cases, while the other two cases had 0.25 cc and 0.27 cc residual tumor.
| Patient | Age(yr) | KPS(%) | RPA | Sex | MMSE score | Tumor volume (cc) | MGMT promoter | Tumor location | PFS (mo) | OS (mo) |
| 1 | 69 | 70 | 5 | M | 28 | 111.8 | Methylated | right frontal | 19.5 | 27.0 |
| 2 | 73 | 70 | 5 | M | 27 | 34.0 | Unmethylated | left temporal | 3.1 | 9.1 |
| 3 | 50 | 80 | 5 | F | 26 | 12.9 | Methylated | left temporal | 3.2 | > 36.0 |
| 4 | 67 | 60 | 5 | F | 28 | 68.3 | Methylated | right frontal | 16.1 | 27.4 |
| 5 | 71 | 90 | 4 | F | 30 | 44.8 | Methylated | right frontal | 20.3 | > 32.0 |
Enough tumor lysate and DC were available in all cases. From three patients (UPN 1, 4 and 5), we separately received material from both central and invasive portions of the tumor. Both tumor components were used for vaccine production in all three cases. In the remaining two cases, only material from the central portion was obtained. The amount of tumor lysate used to pulse the DC of each patient is specified in Table 2.
| Number of producedvaccine doses | Number of administratedvaccine doses | Number of DC/dose | Amount tumor lysate (mg/mL)/107 iDC | ||
| Range | Mean | ||||
| UPN 1 | 16 | 11 | 10.00-10.00 × 106 | 10.00 × 106 | 67.4 (33.7 CP + 33.7 IP) |
| UPN 2 | 18 | 4 | 8.25-9.20 × 106 | 8.85 × 106 | 52.75 (CP) |
| UPN 3 | 4 | 4 | 2.30-6.80 × 106 | 3.40 × 106 | 75 (CP) |
| UPN 4 | 15 | 15 | 0.82-10.00 × 106 | 4.90 × 106 | 75 (37.5 CP + 37.5 IP) / 50 (20 CP + 30 IP) |
| UPN 5 | 16 | 12 | 1.00-10.00 × 106 | 4.89 × 106 | 75 (19 CP + 56 IP) |
For three patients (UPN 1, 2, 5) one leukoapheresis sufficed to produce the amount of DC to be used for several doses (16, 16 and 18, respectively). In one case (UPN 4), two leukoaphereses were instead necessary, since the number of DC obtained allowed the preparation of 4 and 11 vaccine doses, respectively. The fifth patient (UPN 3) underwent only one leukoapheresis, though the production of DC was limited to 4 doses, because at the time of his first DC vaccination radiological signs of a possible progression had already been detected.
The cells used for vaccination are mature DC. They strongly express CD33 as well as CD11c and CD209. Vice versa, they lack CD19, CD3 and CD14 expression and have high expression of HLA-DR, CD40, CD86 and CD83 (data not shown).
UPN 2 received only four vaccine doses because of rapid clinical deterioration, while UPN 3 received only four doses due to the low cell amount obtained through the leukoapheresis. The other patients (UPN 1, 4 and 5) received 11, 15 and 12 vaccine doses, respectively.
UPN 2, 3 and 4 experienced administrative delays and could not receive the first dose prior to radiotherapy. As for UPN 2 and 3, possible early radiological progression signs were detected at the time of the first vaccination. UPN 1, 4 (except for the timing of the first vaccine dose) and 5 followed the vaccination calendar as described above without deviations.
Results are shown in Table 3. PBMC proliferation after stimulation with tumor lysate-pulsed DC was detected in all three patients (UPN 1, 2, 5) in whom the test was performed (Figure 1). In the three cases, autologous PBMC stimulated with tumor lysate-pulsed DC also produced not negligible amounts of IFN-γ (not shown) compared to controls, and UPN 5 did so even when his PBMC were stimulated with tumor lysate alone from both central and invasive portions (not shown). Vice versa, all ELISPOT tests were negative (not shown).
| Patient | Cellular response | |||||||||||
| Proliferation | Cytokines | |||||||||||
| DC | Tumor lysate | IFN-γ | TNF-α | |||||||||
| Centralportion | Invasiveportion | ELISA | ELISPOT | ELISA | ||||||||
| DC | Tumor lysate | DC | Tumor lysate | DC | Tumor lysate | |||||||
| Central portion | Invasive portion | Central portion | Invasive portion | Central portion | Invasive portion | |||||||
| UPN 1 | + | - | ND | + | - | ND | - | - | ND | - | - | ND |
| UPN 2 | + | - | ND | + | - | ND | - | - | ND | - | - | ND |
| UPN 3 | ND | - | ND | ND | - | ND | ND | - | ND | ND | - | ND |
| UPN 4 | ND | - | - | ND | - | - | ND | - | - | ND | - | - |
| UPN 5 | + | - | - | + | + | + | - | - | - | - | - | - |
Neurological deficits after surgery were as follows: one patient (UPN 3) experienced a transient worsening of a right hemiparesis present before surgery; at 6 mo, she still had a 4+/5 right hemiparesis. UPN 2 had a generalized seizure one month after surgery.
Toxicity caused by radio-chemotherapy was in line with normally associated with the corresponding regimens.
Neither adverse events nor toxicity attributable to the immunotherapy were observed.
All patients have progressed, with a median progression-free survival (PFS) from surgery of 16.1 mo (range: 3.1-20.3). Only one patient was re-operated at recurrence. UPN 2 and 3 had already early radiological progression signs at the time of the first vaccination. In both patients, bevacizumab was started after a second MRI indicated that these signs were not suggestive of pseudo-progression. UPN 2 had a partial response before further progression and death. UPN 3 had a total response to bevacizumab.
Median and mean OS were 27.4 mo and 25.1 mo, respectively (Table 1). Four of five patients were alive at two years. The number of circulating leukocytes and lymphocytes at the time of each and every vaccination did not correlate with immune response to vaccination and OS. Three patients have died with OS of 9 mo, 27 mo and 27.4 mo, while the other two are still alive at 29 mo and 33 mo. Both living patients are ambulatory and independent. UPN 5 is still receiving treatment with bevacizumab, with total response, while UPN 3 has not received any treatment over the last 9 mo. Contrast enhancement has reappeared around the surgical bed and has been followed for three mo without any symptom.
All five patients exceeded the OS expected with standard of care calculated with EORTC nomograms[13], with a mean difference of 11.4 mo (25.1-13.7). Median OS expected in a group with these prognostic factors would be 12.3. Yet, the OS of our group exceeded it by 14.7 mo (27 to 12.3). Even with only five patients, the binomial test, was almost significant (P = 0.06). The five patients have also lived longed than the historic controls, median 31.2 vs 11.5, this difference was significant by log-rank (Mantel-Cox), P = 0.02.
This work presents the results of a pilot group of five patients with GBM treated with experimental immunotherapy consisting of autologous DC pulsed with autologous tumor lysate added to standard therapy.
Several groups have attempted a conceptually similar immunotherapeutic approach in patients with GBM[14]. The strategy appears to be feasible, safe, well tolerated and, at least in some instances, potentially beneficial in terms of PFS and OS. On the other hand, there is not yet a clear consensus on the best DC-based vaccine formulation, dose, route of administration and schedule to stimulate the most effective immune response in the largest possible proportion of patients. Some groups prefer to use immature DC[7,15-23], while we and others focus our efforts on mature DC[24-29] .
In our study, the number of DC used for vaccination differs among patients, but we think that, within certain limits, this does not condition the effectiveness of treatment. We simply used the number of DC available in each and every case.
Given our extended experience with idiotype vaccines against B-cell malignancies[30], we are trying to build a solid foundation for our GBM active immunotherapy keeping into account similarities and differences between the two customized strategies. The former deals with an indolent disease, targets a known tumor-specific antigen, has shown biological and clinical efficacy as well as clinical benefit, but has not passed the test of randomized clinical trials[31]. The latter deals with a dreadful disease, targets unknown tumor-associated and/or-specific antigens, has formally shown biological efficacy-e.g., immune responses induced by vaccination, but not yet clinical efficacy and clinical benefit[13]; moreover, it has not yet completed a randomized clinical trial. The fact that clinical efficacy (e.g., the ability to kill tumor cells in vivo) cannot be easily shown in GBM, given the lack of a suitable surrogate marker, is not a major concern as long as we can show clinical benefit, that is improved OS and/or PFS.
Some very long survival times have been reported. However, efficacy is not yet proved. An important issue in previous immunotherapy trials for GBM is the inclusion criteria, as most require the patients to be off steroids after surgery and after radiotherapy[5-7,22], or exclude patients who had radiographic progression after standard radio-chemotherapy[32]. The selection creates important biases in any efficacy comparison, as the patients with very early progression clearly represent the worst cases[33]. We aim at treating a wide base of patients with different ages and functional status, while we also enroll the patients from surgery precisely to avoid these biases.
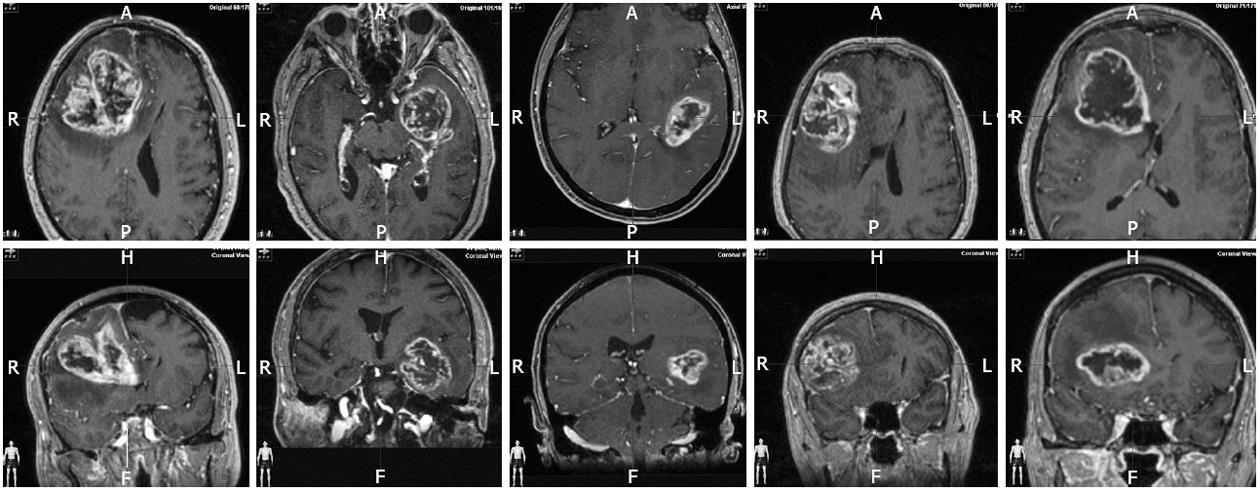
The only limitation of our selection criteria is that we enroll only resectable cases. The amount of residual tumor is also accepted as very important for the survival and for the efficacy of immunotherapy. This study includes fluorescence guided resection and the intention to make a gross total resection as part of the treatment protocol. So, ours is not a group of patients selected a posteriori because they underwent a good resection, but a treatment scheme designed to achieve benefit from the combination of maximal surgery, radiochemotherapy, and immunotherapy. Figure 2 shows representative MRI slides of the five cases to clarify this important point. We have previously shown that with experience in FGS, the inclusion criteria of less than 1cc can be reached in a majority of patients including complex surgical cases[9]. Others have showed similar results with intraoperative MRI[34].
The other difference in our treatment schedule from previous works using immunotherapy added to standard radio-chemotherapy consists in beginning vaccination prior to radio-chemotherapy. We have shown that in the typical 4 wk interval between surgery and radio-chemotherapy there is time to manufacture the vaccines and administer the first dose. We think that this dose can help to promote a faster increase of the response from subsequent doses, even though we do not expect an immediate benefit during the period of immunosuppression induced by radiochemotherapy.
The patient sample presented here is not selected by young age, good functional status or absence of early progression. Therefore, it is representative of a wide population of GBM patients; 80% were in RPA class V and mean age was 67 Patients with first-line immunotherapy in the work of Prins et al[5] had a mean age of 49.7 years and 60% were RPA class III, while in the work of Ardon et al[6] mean age was 50.3 and 7 out of 8 patients were RPA IV, and one RPA III.
Despite the small number of cases, the survival time is clearly unexpected for a group with these characteristics. MGMT promoter methylation was the only positive prognostic factor, and yet the cohort with methylated MGMT promoter in the EORTC-NCIC shows a median OS of 23 mo[2], while all of our 4 methylated patients have lived longer than 27 mo, with two still alive. The median OS in our group exceeds the OS expected using the EORTC nomograms[13] by almost 15 mo (27.0 to 12.3). Even with the intrinsic limitations of the small sample size, we conclude that this DC-based immunotherapy is likely to have added to the results of standard of care and provided an OS clearly superior to the expectations, suggesting a strong benefit from immunotherapy in an unfavorable group of patients.
This work is dedicated to the memory of Dr Javier Pérez Calvo, who over the years was an instrumental driving force of the overall program and who sadly died in August 2012 after fighting against leukemia since January 2011.
Glioblastoma multiforme is the most frequent brain tumor and is all but incurable. Even with the most sophisticated surgical approaches, including fluorescence-guided complete or near complete resection, followed by standard of care the typical survival does not go beyond 12-15 mo.
Tumor lysate-pulsed dendritic cell vaccination is an experimental type of customized, active immunotherapy currently under development. It aims at educating the patient’s immune system in order to prevent tumor reoccurrence/progression. As such, it is meant to be administered shortly after the maximum response to previous therapies.
In this pilot study, the authors enrolled five consecutive glioblastoma patients with poor prognosis. In most cases, they would have not been enrolled in the previous clinical trials mentioned above. Yet, following fluorescence-guided surgery and tumor lysate-pulsed dendritic cell vaccination, they all experienced remarkable progression-free survivals. In a couple of cases, this result is still ongoing over two years after surgery.
The study results suggest that tumor lysate-pulsed dendritic cell vaccination, after fluorescence-guided complete or near complete surgery, should be attempted in all resectable patients with glioblastoma multiforme, not only in those with better prognosis.
Fluorescence - guided surgery: it is a surgical technique enhancing the ability of the neurosurgeon to remove nearly all the tumor by a better visualization of the tumor resection margins; tumor lysate: it is the product of mechanical disaggregation of the tumor removed during surgery; Dendritic cells: autologous - therefore patient - specific - antigen presenting cells which are derived from monocytes and matured in vitro.
Despite the small number of patients enrolled in this pilot study, the results of this treatment are extremely promising and warrant expansion in the currently ongoing, larger clinical trial.
Peer reviewers: Luis F Porrata, MD, Assistant Professor, Department of Hematology and Internal Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, United States; Haval Shirwan, PhD, Professor and Hamilton Endowed Chair in Autoimmunity, Chair in Autoimmune Disease, Director, Molecular Immunomodulation Program, Institute for Cellular Therapeutics, Baxter Bldg. I, Suite 404E, 570 South Preston St., University of Louisville, KY40202-1760, United States
S-Editor Jiang L L-Editor A E-Editor Lu YJ
| 1. | Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D, Farina R, Farinotti M, Fariselli L, Finocchiaro G. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443-2449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 360] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 4. | Li J, Wang M, Won M, Shaw EG, Coughlin C, Curran WJ, Mehta MP. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81:623-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 6. | Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, Demaerel P, Bijttebier P, Claes L, Goffin J. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955-5964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Díez Valle R, Tejada Solis S, Idoate Gastearena MA, García de Eulate R, Domínguez Echávarri P, Aristu Mendiroz J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol. 2011;102:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Idoate MA, Díez Valle R, Echeveste J, Tejada S. Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology. 2011;31:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109. [PubMed] |
| 12. | Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2528] [Cited by in RCA: 2908] [Article Influence: 193.9] [Reference Citation Analysis (0)] |
| 13. | Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K, Brandes AA, Allgeier A. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kim W, Liau LM. Dendritic cell vaccines for brain tumors. Neurosurg Clin N Am. 2010;21:139-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Liau LM, Black KL, Martin NA, Sykes SN, Bronstein JM, Jouben-Steele L, Mischel PS, Belldegrun A, Cloughesy TF. Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report. Neurosurg Focus. 2000;9:e8. [PubMed] |
| 16. | Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842-847. [PubMed] |
| 17. | Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, Lee PK. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927-4933. [PubMed] |
| 19. | Kobayashi T, Yamanaka R, Homma J, Tsuchiya N, Yajima N, Yoshida S, Tanaka R. Tumor mRNA-loaded dendritic cells elicit tumor-specific CD8(+) cytotoxic T cells in patients with malignant glioma. Cancer Immunol Immunother. 2003;52:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, Downie P, Hassall TE, Tang ML, Ashley DM. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 2004;6:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973-4979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 751] [Reference Citation Analysis (0)] |
| 22. | Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515-5525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337-344. [PubMed] |
| 25. | Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, Kufe DW, Ohno T. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452-459. [PubMed] |
| 26. | Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JE, Kühl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Plets C. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | De Vleeschouwer S, Van Calenbergh F, Demaerel P, Flamen P, Rutkowski S, Kaempgen E, Wolff JE, Plets C, Sciot R, Van Gool SW. Transient local response and persistent tumor control in a child with recurrent malignant glioma: treatment with combination therapy including dendritic cell therapy. Case report. J Neurosurg. 2004;100:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160-4167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 447] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 30. | Bendandi M, Rodríguez-Calvillo M, Inogés S, López-Díaz de Cerio A, Pérez-Simón JA, Rodríguez-Caballero A, García-Montero A, Almeida J, Zabalegui N, Giraldo P. Combined vaccination with idiotype-pulsed allogeneic dendritic cells and soluble protein idiotype for multiple myeloma patients relapsing after reduced-intensity conditioning allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Bendandi M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer. 2009;9:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722-4729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 33. | Pirzkall A, McGue C, Saraswathy S, Cha S, Liu R, Vandenberg S, Lamborn KR, Berger MS, Chang SM, Nelson SJ. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2009;11:842-852. [PubMed] |
| 34. | Hatiboglu MA, Weinberg JS, Suki D, Rao G, Prabhu SS, Shah K, Jackson E, Sawaya R. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64:1073-1081; discussion 1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |