Copyright
©The Author(s) 2024.
World J Clin Oncol. Aug 24, 2024; 15(8): 968-974
Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.968
Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.968
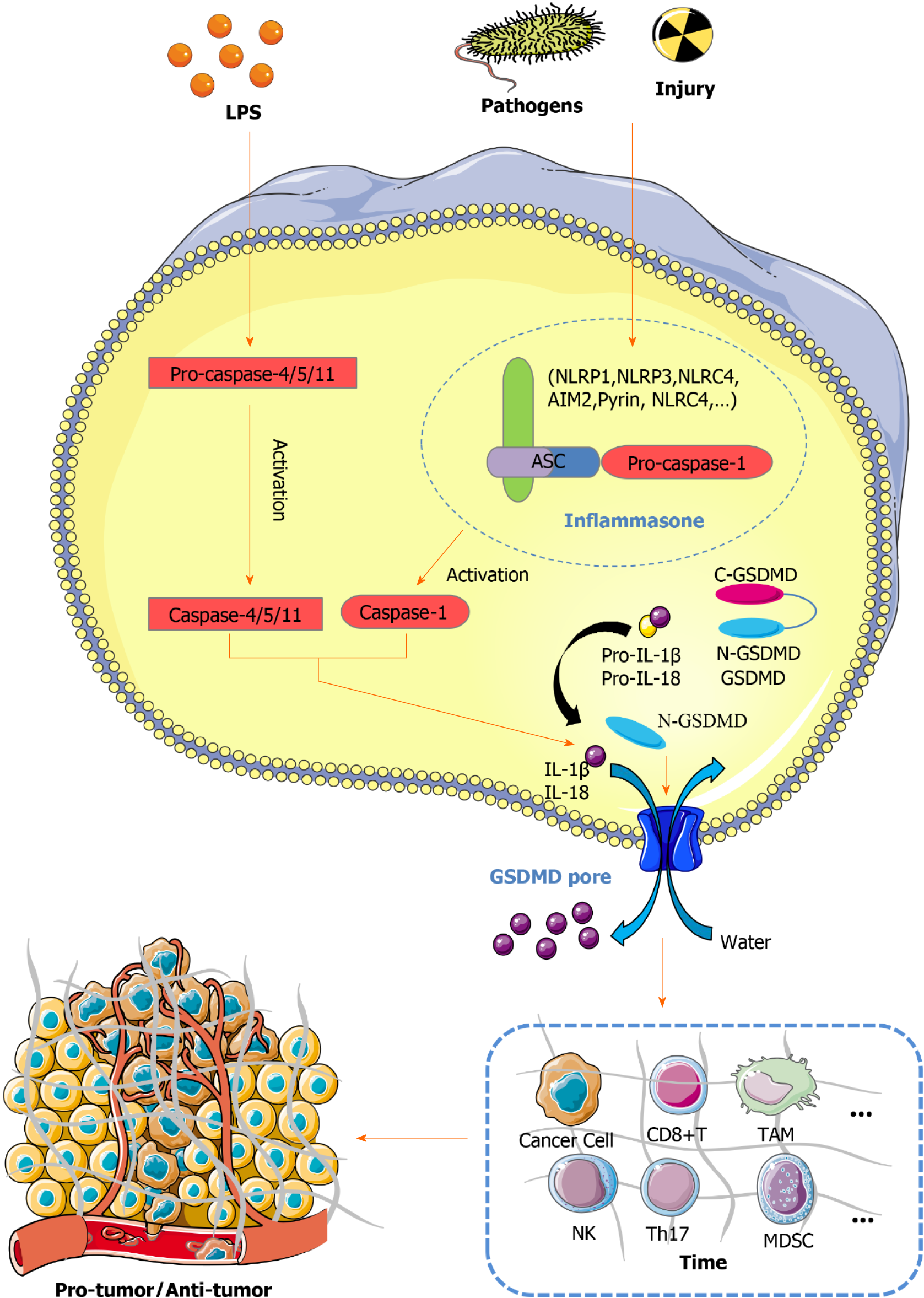
Figure 1 Role of pyroptosis in regulating the tumor immune microenvironment and tumor growth.
In the canonical pathway, inflammasomes recognize exogenous pathogens and endogenous damage. The intracellular sensor proteins NLRP1, NLRP3, NLRC4, AIM2, Pyrin, and NLRC4 recruit ASC and then activate caspase-1. In the noncanonical pathway, lipopolysaccharide can activate caspase-4/5/11. Activated caspase-1/4/5/11 can cleave GSDMD to release N-GSDMD and pro-IL-1β/18, promoting their maturation. On one hand, N-GSDMD can form nonselective pores, further causing water influx, lysis, and death. Additionally, IL-1β and IL-18 can be released via these pores. On the other hand, N-GSDM, IL-1β, and IL-18 can affect immune cells such as cancer cells in the tumor immune microenvironment, tumor-associated macrophages, CD8+ T cells, and NK cells. Therefore, pyroptosis is a complex regulatory network that acts as a bridge between the immune system and tumors and as a double-edged sword in the tumor immune microenvironment. In this way, it influences tumorigenesis, leading to protumor effects and antitumor effects. LPS: Lipopolysaccharide; TIME: Tumor immune microenvironment.
- Citation: Wang JY, Lu YH, Li F, Huang ML. Pyroptosis: A promising biomarker for predicting colorectal cancer prognosis and enhancing immunotherapy efficacy. World J Clin Oncol 2024; 15(8): 968-974
- URL: https://www.wjgnet.com/2218-4333/full/v15/i8/968.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i8.968