Copyright
©The Author(s) 2022.
World J Clin Oncol. Nov 24, 2022; 13(11): 866-879
Published online Nov 24, 2022. doi: 10.5306/wjco.v13.i11.866
Published online Nov 24, 2022. doi: 10.5306/wjco.v13.i11.866
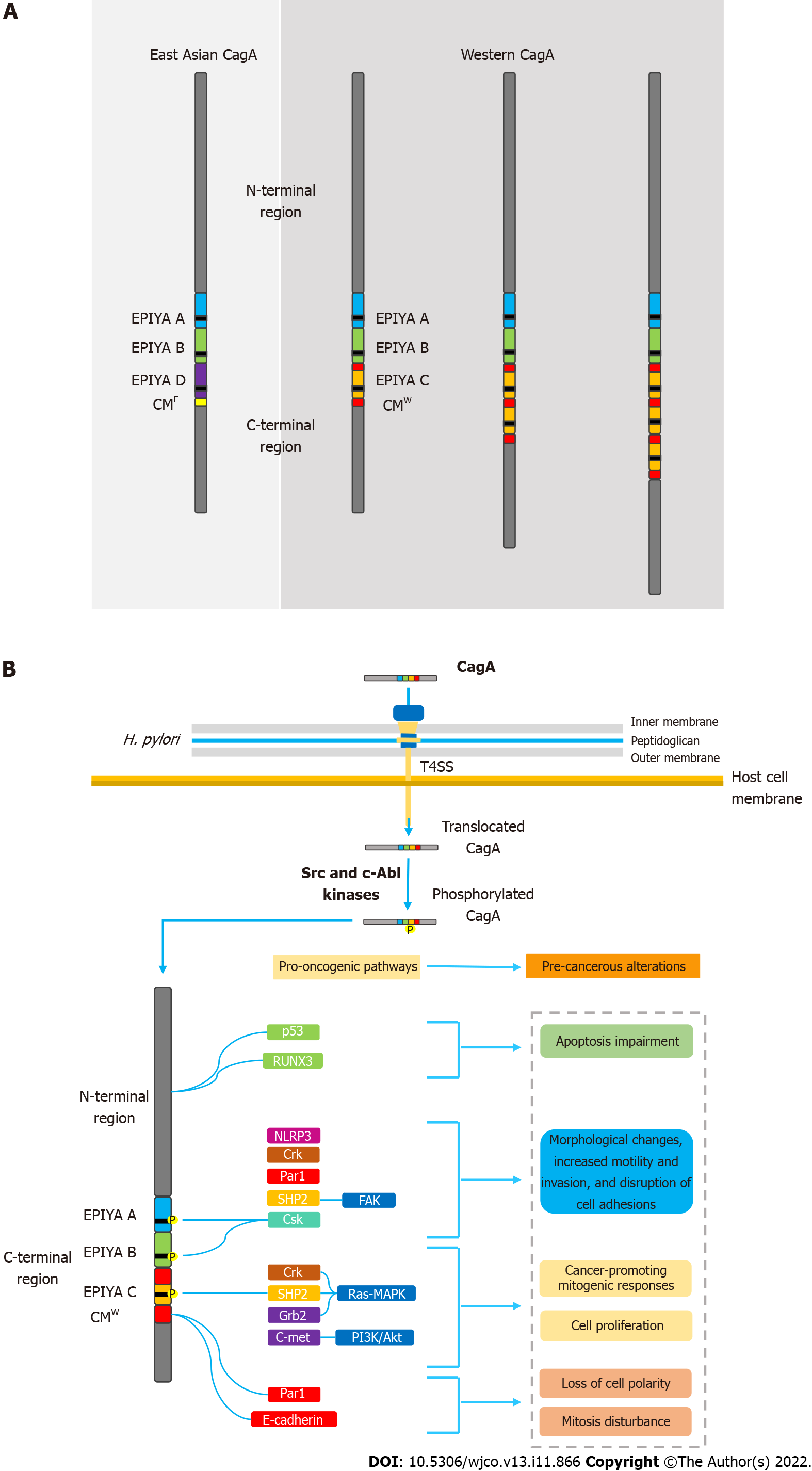
Figure 1 Helicobacter pylori oncoprotein: molecular structure and CagA-mediated carcinogenesis underlying mechanisms.
A: Structural domain differences between East Asian and western CagA. CagA tertiary structure is characterized by a structured N-terminal region and an unstructured C-terminal tail. The oncoprotein contains repetitive sequences in its C-terminal polymorphic region, known as the EPIYA motifs and CM motif. EPIYA-A and EPIYA-B motifs were identified in almost all CagA-positive H. pylori strains, followed by one, two, or three EPIYA-C sequences in western strains, or an EPIYA-D segment in East Asian strains. The CM motif, although highly conserved, possesses a 5-amino-acid difference between East Asian and western strains, hence distinguishing East Asian and Western CagA; B: Molecular mechanisms of CagA-mediated carcinogenesis. Upon translocation, CagA EPIYA motifs are tyrosine-phosphorylated by Src family or c-Abl kinases of the host cell. After phosphorylation, CagA localizes to the inner leaflet of the plasmatic membrane and acts as a promiscuous scaffold or hub protein that simultaneously disturbs multiple host signaling pathways, involved in regulation of a large range of cellular processes, including proliferation, differentiation and apoptosis. Ultimately, the disharmonic interaction between CagA and host proteins leads to pro-oncogenic cellular alterations. CM: CagA-multimerization; CMW: western CagA.
- Citation: Freire de Melo F, Marques HS, Rocha Pinheiro SL, Lemos FFB, Silva Luz M, Nayara Teixeira K, Souza CL, Oliveira MV. Influence of Helicobacter pylori oncoprotein CagA in gastric cancer: A critical-reflective analysis. World J Clin Oncol 2022; 13(11): 866-879
- URL: https://www.wjgnet.com/2218-4333/full/v13/i11/866.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i11.866