Copyright
©The Author(s) 2021.
World J Clin Oncol. Aug 24, 2021; 12(8): 656-663
Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.656
Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.656
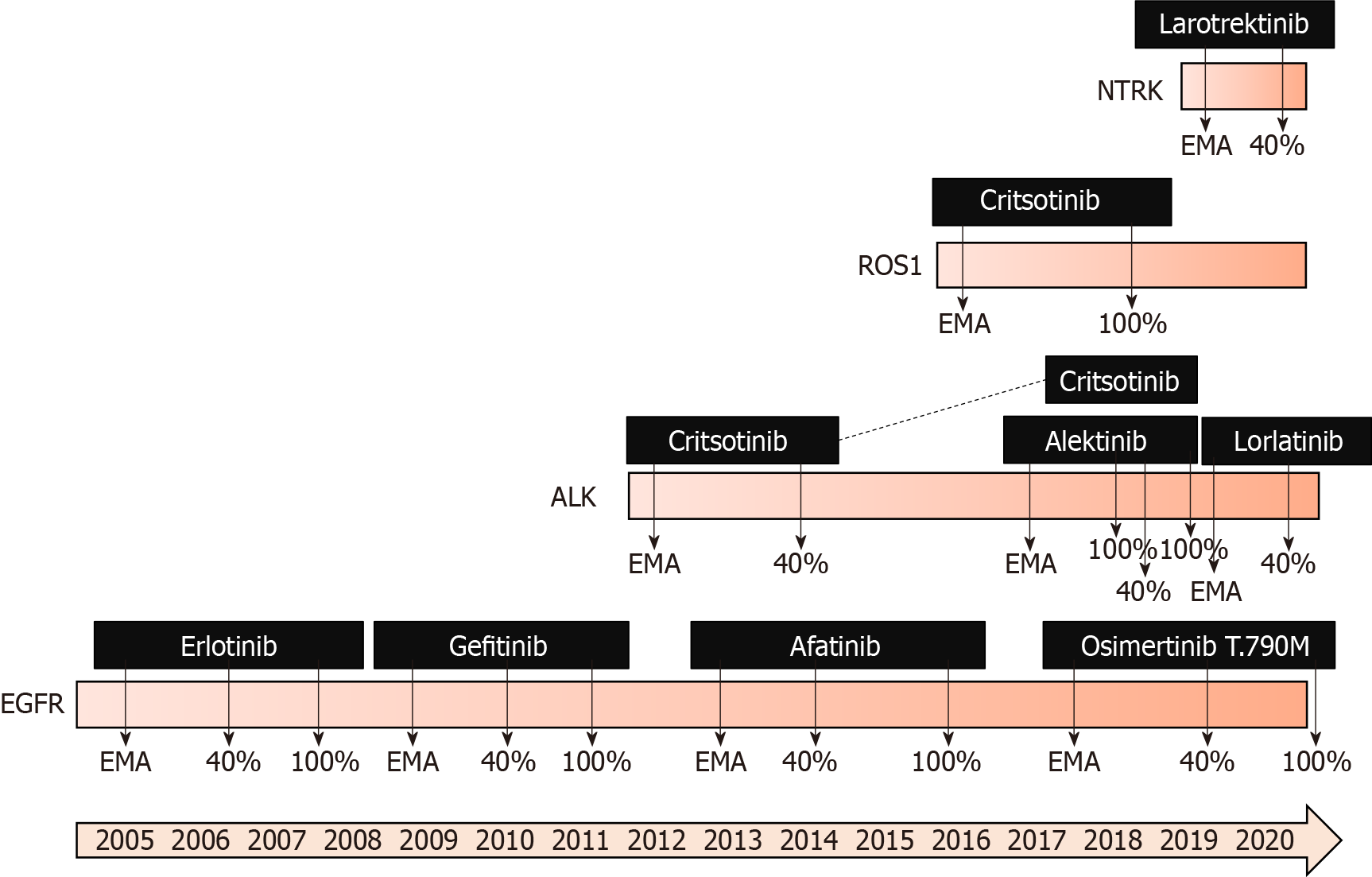
Figure 2 Presenting the history of the European Medicines Agency approvals and reimbursement situation in Finland (40% reimbursement and 100% reimbursement), concerning gene-driven therapies for lung cancer.
NTRK: Neurotrophic receptor tyrosine kinase; PET: Positron emission tomography; CT: Computed tomography; EMA: European Medicines Agency; ALK: Anaplastic lymphoma kinase; EGFR: Epidermal growth factor receptor.
- Citation: Saarenheimo J, Andersen H, Eigeliene N, Jekunen AP. Current challenges in applying gene-driven therapies in clinical lung cancer practice. World J Clin Oncol 2021; 12(8): 656-663
- URL: https://www.wjgnet.com/2218-4333/full/v12/i8/656.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i8.656