Copyright
©The Author(s) 2017.
World J Gastrointest Pharmacol Ther. Feb 6, 2017; 8(1): 47-59
Published online Feb 6, 2017. doi: 10.4292/wjgpt.v8.i1.47
Published online Feb 6, 2017. doi: 10.4292/wjgpt.v8.i1.47
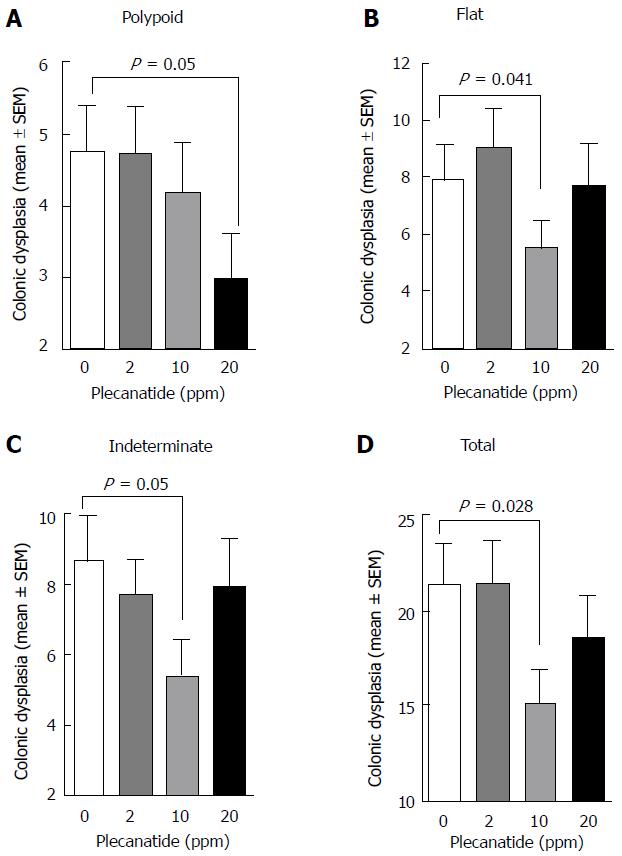
Figure 2 Treatment with plecanatide inhibits inflammation-associated colonic dysplasia in dextran sodium sulfate-treated Apc+/Min-FCCC mice.
Analyses revealed the number of pathologically confirmed polypoid (A), flat (B), indeterminate (C) and total (D) dysplasias within the colon of DSS-treated mice following administration (7 wk) of either control diet or diet supplemented with varying doses of plecanatide (n = 23/group). Wilcoxon 2-sample test and analysis of variance (ANOVA) were used to compare the multiplicity of dysplasias in independent groups. A P value ≤ 0.05 was considered statistically significant. DSS: Dextran sodium sulfate
- Citation: Chang WCL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, Palejwala VA, Clapper ML, Shailubhai K. Plecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc+/Min-FCCC mice. World J Gastrointest Pharmacol Ther 2017; 8(1): 47-59
- URL: https://www.wjgnet.com/2150-5349/full/v8/i1/47.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v8.i1.47