Copyright
©The Author(s) 2016.
World J Gastrointest Pharmacol Ther. May 6, 2016; 7(2): 274-282
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.274
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.274
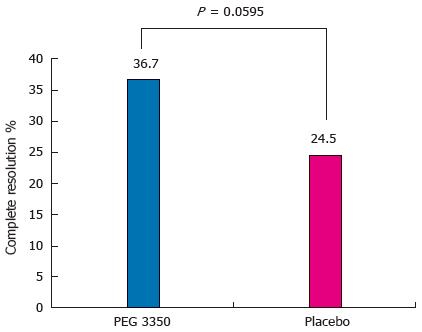
Figure 2 Assessment of primary outcome between polyethylene glycol 3350 and placebo (per protocol population).
Resolution was recorded if the subject reported no occurrence of two or more consecutive unsuccessful bowel movements (BMs) for the rest of the study following the first successful BM. PEG: Polyethylene glycol.
- Citation: McGraw T. Polyethylene glycol 3350 in occasional constipation: A one-week, randomized, placebo-controlled, double-blind trial. World J Gastrointest Pharmacol Ther 2016; 7(2): 274-282
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/274.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.274