Copyright
©The Author(s) 2016.
World J Gastrointest Pharmacol Ther. May 6, 2016; 7(2): 274-282
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.274
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.274
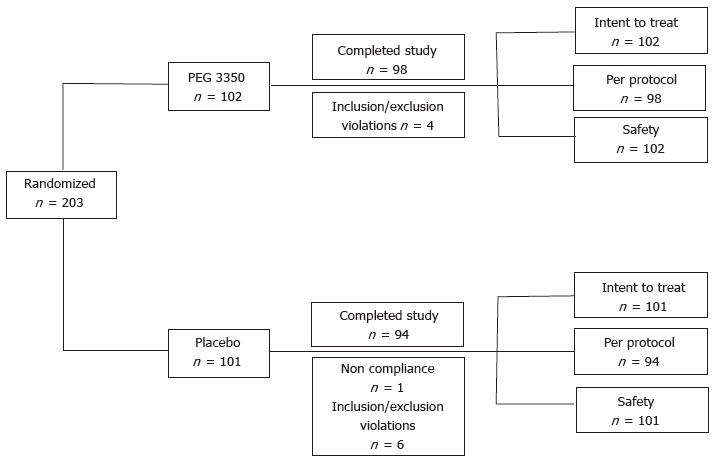
Figure 1 Disposition of subjects.
The ITT population included all subjects randomized to a study treatment and receiving at least one dose of the assigned drug. The per-protocol population included all ITT subjects who additionally exhibited no major protocol violations or other events considered biasing the study outcome. The safety population included all subjects who received one or more dose of the study medication. ITT: Intent-to-treat; PEG: Polyethylene glycol.
- Citation: McGraw T. Polyethylene glycol 3350 in occasional constipation: A one-week, randomized, placebo-controlled, double-blind trial. World J Gastrointest Pharmacol Ther 2016; 7(2): 274-282
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/274.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.274