Copyright
©The Author(s) 2015.
World J Gastrointest Pharmacol Ther. Nov 6, 2015; 6(4): 213-222
Published online Nov 6, 2015. doi: 10.4292/wjgpt.v6.i4.213
Published online Nov 6, 2015. doi: 10.4292/wjgpt.v6.i4.213
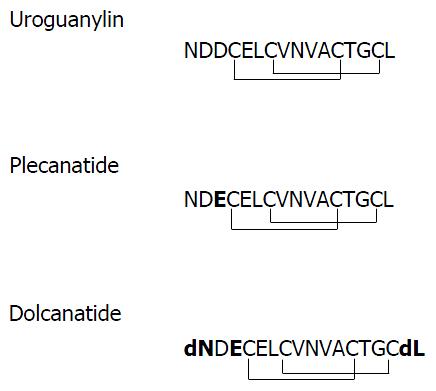
Figure 1 Primary structures of uroguanylin, plecanatide, and dolcanatide.
Single-letter abbreviations for amino acids are depicted. Plecanatide is similar to UG except for the substitution of aspartic acid (D) at position 3 from the N-terminus of UG with glutamic acid (E). The structure of dolcanatide is similar to plecanatide except that the amino acids at both termini are replaced with their respective D-stereoisomers. Uroguanylin as well as its analogs have four cysteines (C) enabling the formation of two intramolecular disulfide bonds. Substituted amino acids in plecanatide and dolcanatide are shown in bold type. UG: Uroguanylin.
- Citation: Shailubhai K, Palejwala V, Arjunan KP, Saykhedkar S, Nefsky B, Foss JA, Comiskey S, Jacob GS, Plevy SE. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther 2015; 6(4): 213-222
- URL: https://www.wjgnet.com/2150-5349/full/v6/i4/213.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i4.213