Copyright
©The Author(s) 2015.
World J Gastrointest Pharmacol Ther. Aug 6, 2015; 6(3): 32-58
Published online Aug 6, 2015. doi: 10.4292/wjgpt.v6.i3.32
Published online Aug 6, 2015. doi: 10.4292/wjgpt.v6.i3.32
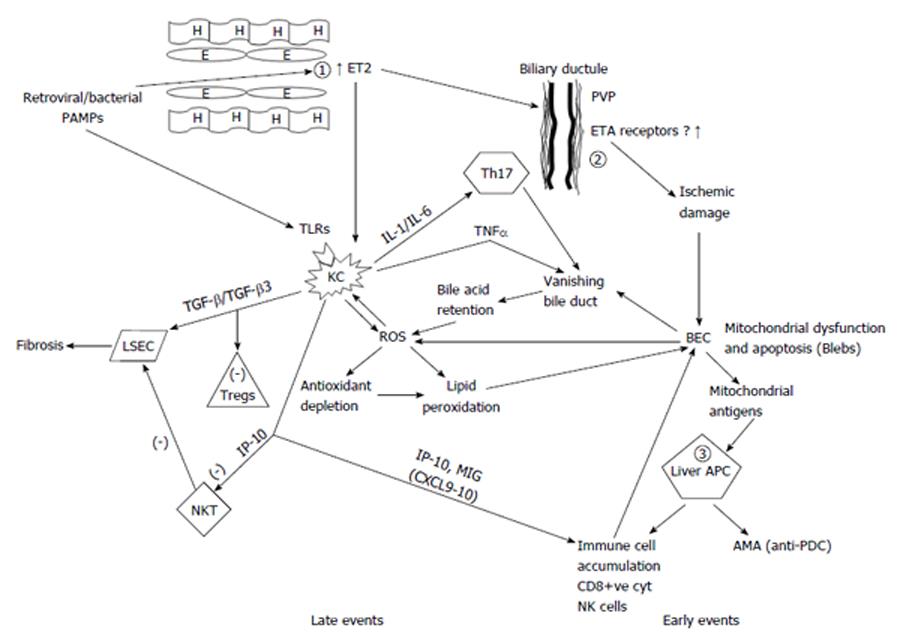
Figure 1 Liver sinusoidal endothelial cells and Kupffer cells are mandatory in the pathogenesis of primary biliary cirrhosis with early and late events in the unifying model.
The fundamental early defect in primary biliary cirrhosis (PBC) is the overproduction of endothelin 2 (ET2) by endothelial cells (1) possibly driven by a retrovirus or other microbial pathogens in genetically predisposed individuals. ET2 is a chemoattractant for Kupffer cells and also causes contraction of stellate cells leading to early portal hypertension. ET2 leads to ischemic damage of biliary epithelial cells (BEC) (2) through constriction of the peribiliary vascular plexus with resultant BEC mitochondrial dysfunction and eventually apoptosis leading to the vanishing bile duct syndrome. Apoptotic blebs containing immunologically active mitochondrial antigens possibly generated through caspase cleavage, are presented by hyper-responsive liver dendritic cells (APC) (3) and lead to immune cell accumulation and antimitochondrial antibody production. The second fundamental defect occurring later in the disease process is the production of reactive oxygen species (ROS) driven by both bile acid accumulation as a result of the vanishing bile ducts and generation by Kupffer cells through ingestion of BEC apoptotic bodies. ROS drives the accumulated Kupffer cells to produce more ROS and pro-inflammatory cytokines. Moreover, a relative antioxidant insufficiency allows ROS to cause lipid peroxidation and further BEC apoptosis and mitochondrial dysfunction. CXCL chemokines produced by endothelial and Kupffer cells drive the accumulation of various immune cells while IP-10 inhibits apoptosis of LSCs induced by natural-killer T (NKT)-cells and thus favors the fibrosis induced by transforming growth factor (TGF)-β produced by Kupffer cells. However an increased TGF-β3 production may also explain the reduced Tregs found in PBC. In this model BECs are destroyed by other mechanisms in addition to the initial ischemia. ROS damage, NK cell killing induced by interferon alpha (IFNα) production by Kupffer cells, cytotoxicity by CD8+ve cells and T-helper 17 (Th17) cell, are also implicated. All these lead to the development of the vanishing bile duct syndrome characteristic of PBC. H: Hepatocyte; E: Endothelial cell; ET2: Endothelin 2; PVP: Peribiliary vascular plexus; PAMP: Pathogen-associated molecular patterns; ETA: Endothelin-A; TLR: Toll-like receptors; IL: Interleukin; TNFα: Tumor necrosis factor alpha; KC: Kupffer cells; TGF-β: Transforming growth factor beta; BEC: Biliary epithelial cells; ROS: Reactive oxygen species; LSEC: Liver sinusoid endothelial cells; Tregs: Regulatory T-cells; IP-10: Interferon gamma-induced protein 10; APC: Antigen presenting cells; MIG: Monokine induced by gamma interferon; NKT: Natural killer T-cells; Anti-PDC: Anti-pyruvate decarboxylase antibodies; AMA: Antimitochondrial antibodies.
- Citation: Kouroumalis E, Notas G. Primary biliary cirrhosis: From bench to bedside. World J Gastrointest Pharmacol Ther 2015; 6(3): 32-58
- URL: https://www.wjgnet.com/2150-5349/full/v6/i3/32.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i3.32