INTRODUCTION
There are over 500000 patients hospitalized annually for gastrointestinal bleeding (GIB) in the United States, which carries an inpatient mortality rate of 3%[1]. Patients presenting with GIB are considered as having either overt or occult GIB, depending upon the presence of visible or non-visible bleeding[2]. Overt GIB presents as visible bleeding which constitutes melena, hematochezia or hematemesis. Occult GIB manifests as positive immunohistochemical staining or as iron deficiency anemia[3]. Irrespectively, the initial evaluation of either overt or occult GIB includes an esophagoduodeonscopy and/or colonoscopy. Obscure GIB (OGIB) refers to the patient population with persistent or recurrent GIB where the initial endoscopic evaluation was negative, estimated to be the case in approximately 5% of patients[3]. As a result, these patients undergo extensive testing with the objective of localizing and potentially treating the bleeding lesion. Estimates suggest that an average of $33630 is spent per Medicare patient for further evaluation of OGIB[4]. While radiologic advances have changed the approach to GIB, the lack of an algorithmic approach and understanding of the available modalities have made it difficult for clinicians to practice cost-effective medicine. Diagnostic and therapeutic modalities differ in the setting of upper GIB (UGIB) vs lower GIB (LGIB). This article is intended to present the newest radiologic modalities available as it pertains to UGIB and LGIB as well as provide an algorithmic approach to GIB and the role of endoscopic markings.
UPPER GI BLEED
Approximately 100000 are patients hospitalized annually for an UGIB, which carries a mortality rate as high as 11%[11]. The cornerstone for diagnosis and therapeutic intervention in UGIB revolves around endoscopy. There are no radiologic approaches that have demonstrated utility in the diagnosis of an UGIB. There are, however, radiologic interventions that may be used when endoscopic therapy has proven futile, namely transjugular intrahepatic portosystemic shunt, retrograde transvenous obliteration, and transcatheter selective embolization.
Transjugular intrahepatic portosystemic shunt
Transjugular intrahepatic portosystemic shunts (TIPS) utilizes angiographic guidance to create a low-resistance communication between the hepatic vein and the intrahepatic portion of the portal vein[12]. The expandable metal stent maintains patency of the tract allowing for continued systemic circulation. The indication for TIPS in the setting of an UGIB include acute esophageal, gastric, or ectopic variceal hemorrhages and in the prevention of recurrent variceal bleeding.
García-Pagán et al[13] evaluated the use of TIPS in 63 patients with cirrhosis (Child-Pugh class B or C) presenting with persistent bleeding. Patients were randomly assigned to treatment with TIPS (early-TIPS; n = 32) or continuation of vasoactive therapy with propranolol or nadolol and long-term endoscopic band ligation (pharmacotherapy-EBL; n = 31) with future consideration of TIPS as rescue therapy if necessary. Re-bleeding or persistent bleeding occurred in 14 patients in the pharmacotherapy-EBL group as compared to 1 patient in the early-TIPS group during a median follow up at 16 mo (P = 0.001). The 1-year likelihood of remaining free from re-bleeding or persistent bleeding was 50% in the pharmacotherapy-EBL group compared 97% in the early-TIPS group (P < 0.001). The 1-year survival in the pharmacotherapy-EBL group compared to the early-TIPS group was 61% and 86%, respectively, which led the authors to conclude that early use of TIPS was associated with significant reduction in treatment failure and mortality in patients with cirrhosis hospitalized for acute variceal bleeding. A retrospective study in 2013 validated these results confirming a favorable 1-year mortality in patients who received early-TIPS when compared to those who received vasoactive and endoscopic therapy at 86% and 70% respectively (P = 0.056)[14].
It has recently been postulated that the use of embolotherapy may further prevent recurrent variceal bleeding and stent dysfunction following TIPS creation[15]. A recent prospective study randomized 106 patients with cirrhosis and recurrent variceal bleeding to TIPS in conjunction with prior embolotherapy via the jugular vein (TIPS + E, n = 54) or TIPS alone (n = 52). The 6-mo rate of shunt patency and overall rate of recurrent variceal bleeding was significantly lower in the TIPS-E cohort as compared those patients who received TIPS alone. The 3-year cumulative rates of shunt patency, recurrent variceal bleeding and mortality were not statistically significant amongst the two groups, however, leading the authors to conclude that the TIPS-E regimen may reduce the rate of recurrent variceal bleeding during the first 6 mo but the long term benefit requires further investigation.
Retrograde transvenous obliteration
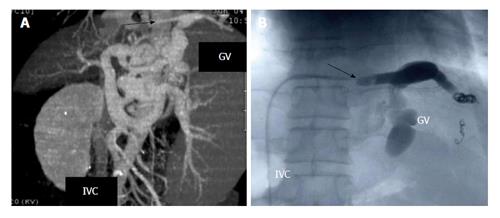
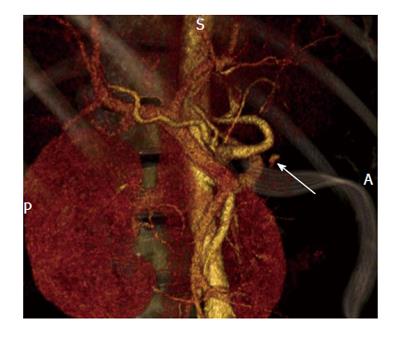
Gastric varices are an important manifestation of portal hypertension and are associated with a very high mortality rate approaching 55%[16]. Originating from short gastric and gastroepiploic veins, cardiofundal gastric varices present with a unique vascular anatomy as they have a tendency to develop spontaneous splenorenal or gastrorenal shunts[17]. Thus the use of cyanoacrylate via endoscopic sclerotherapy on gastric varices carries a risk of systemic migration through the inferior vena cava. TIPS has been performed for this reason, however is only associated with a success rate of 50% in the regression of gastric varices and is associated with an elevated risk for the development or exacerbation of underlying hepatic encephalopathy[18]. As a result, a new minimally invasive procedure known as retrograde transvenous obliteration (B-RTO) has been developed. This technique utilizes a sclerosing agent to thrombose gastric varices via a balloon catheter typically introduced through the femoral vein and left renal. Figure 1 depicts the use of B-RTO in thrombosing gastric varices.
Figure 1 Balloon retrograde transvenous obliteration[59].
A: Multidimensional computed tomography revealing varices supplied by the left gastric vein (GV) and subsequent drainage into the subphrenic vein (illustrated by the arrow) which is connected to the inferior vena cava (IVC); B: Fluoroscopic image of B-RTO depicting placement of the occlusive balloon catheter into the bleeding vessel (demonstrated by the arrow). B-RTO: Balloon-occluded retrograde transvenous obliteration.
A recent meta-analysis evaluated the outcomes for B-RTO for gastric varices[19]. A total of 20 suitable studies were included totaling 734 patients from multiple centers. Cumulative rates for gastric variceal recurrence were reported to be 2.6% (0%-7.2%) with 1-year, 3-year, and 5-year survival rates to be 92.2% (83.1%-100%), 82.6% (71%-100%), and 67.9% (53.7%-85%), respectively. This led the authors to conclude B-RTO should be considered in the management of gastric varices when a gastrorenal shunt is present. Several other retrospective studies have demonstrated technical success with B-RTO concluding it to be effective in obliterating bleeding gastric varices with good short-term outcomes[20-22]. The use of ethanolamine oleate as a sclerosant when performing B-RTO should be with caution as recent studies have implicated it in pulmonary function disorders[23]. The total amount of ethanolamine oleate has been shown to have a direct correlation to the extent of pulmonary damage, and thus careful respiratory monitoring may be necessary in patients undergoing B-RTO, particularly when anticipating the use of large volumes of ethanolamine oleate.
Transcatheter selective embolization
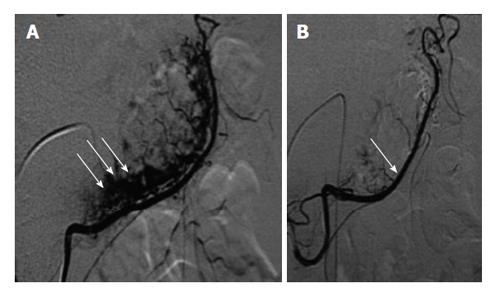
Rösch et al[24] first introduced the use of transcatheter arterial embolization (TAE) in 1972 as an alternative to surgery. The advent of metallic coils, gelfoam, and surgical glue has greatly improved clinical outcomes. The procedure entails a transfemoral or brachial approach with introduction of a 5-french sheath into the common femoral artery. Subsequent arteriography is performed to delineate the anatomy and identify the culprit lesion via contrast extravasation. In the event that the initial approach does not yield a culprit, then superselective catheterization of the gastroduodenal artery, left gastric artery, or splenic artery is performed depending on clinical suspicion[25]. Once the lesions are identified then operator has a plethora of embolization techniques at his disposal, depending on the clinical scenario. The choice of the best embolic agent, however, remains an area of debate. Figure 2 depicts the use of TAE in a gastrointestinal (GI) bleed.
Figure 2 Selective transarterial embolization[60].
A: Selective catheterization of the right gastro epiploic artery in the setting of angiodysplasia. The arrows depict the pathologic vessels at the greater curvature of the stomach; B: Post embolization angiogram revealing control of the bleeding vessel.
Recently, there have been several retrospective analyses comparing the efficacy of TAE to surgery as salvage therapy following failed endoscopic therapy in the setting of UGIB[26,27]. These studies found TAE to be efficacious in controlling life threatening bleeds from an UGI source in high-risk patients who would otherwise be subjected to emergency surgery, which carries a substantially higher mortality rate (nearly 5 times) when compared directly to TAE.
Intra-arterial vasopressin infusion
Vasopressin elicits contractions of smooth muscles in mesenteric vasculature, thus decreasing perfusion pressure to the bowel and potentially thrombosing the bleeding site[28]. Vasopressin is directly infused into the suspected artery at a rate of 0.2-0.4 U/min until control of bleeding is observed on angiography. Once control is documented, vasopressin is infused into the mesenteric artery for another 12-48 h depending on severity.
A review of the literature demonstrated an initial success rate of 70%-80% with use of intra-arterial vasopressin infusion[28]. Unfortunately, the rate of re-hemorrhage with refractory bleeding ranged from 20%-40%. There were various theories that emerged regarding vasopressin failure, namely the rich collateral supply to the upper GI tract, however the exact reason remains unproven. At present time, the emergence of embolotherapy has put vasopressin infusion out of favor.
The bottom line
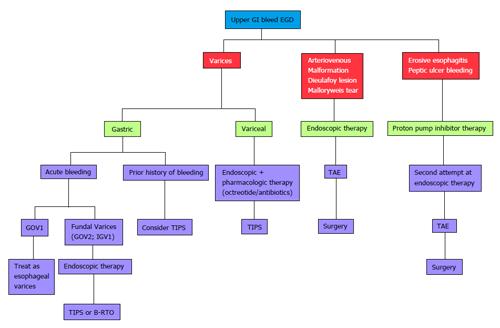
Endoscopy is the point of focus in identifying the culprit of an UGIB and in delivering initial treatment. There are no radiologic modalities that have been proven to show diagnostic utility in the setting of UGIB. When endoscopy proves unsuccessful, however, there are several radiologic alternatives that can potentially treat the underlying lesion including TIPS, B-RTO, and TAE. Figure 3 provides an algorithmic approach to a patient with UGIB.
Figure 3 Algorithmic approach to upper gastrointestinal bleeding.
EGD: Esophagoduodeonscopy; TAE: Transcatheter arterial embolization; TIPS: Transjugular intrahepatic portosystemic shunt; GOV1: Gastroesophageal Varices 1; B-RTO: Balloon-occluded retrograde transvenous obliteration; GI: Gastrointestinal; IGV: Isolated gastric varices.
LOWER GI BLEED
The incidence of LGIB is approximately 20 per 100000 persons[29] with an associated all cause mortality of 3.9%[30]. In addition, patients who experience LGIB during a hospitalization for another condition seem to have a higher mortality when compared to those who are admitted with LGIB as their chief complaint[31]. The differential diagnosis of LGIB ought to include UGIB being that 10%-15% of patients with severe hematochezia are found to have an upper GI source[32]. Hemorrhoidal bleeding is the most common cause of LGIB followed by diverticular bleeding, and next by vascular ectasia[33]. This section will dissect the various tools available for approaching a patient with a LGIB.
Technetium-labeled red blood cell bleeding scan
Technetium-labeled red blood cell bleeding scan (RBC scan), or scintigraphy, is typically the first approach utilized in the localization of an active LGIB. Red blood cells tagged with technetium (99 mTc) are re-injected into the patient with sequential monitoring for extravascular activity. Frequently, images are obtained over 30 to 90 min, and then, if necessary, every few hours for up to 24 h.
Brunnler et al[34] performed a retrospective analysis of 92 patients evaluating the role of technetium-labeled red cell bleeding scan in patients with OGIB. Scintigraphy was able to demonstrate a positive result in all cases, with a false positive rate of only 4%. Notably, a heparin provocation test (a diagnostic approach to localizing the lesion in OGIB which will be later discussed in further detail) increased the diagnostic yield by 46% in patients with a primary negative scan. Scintigraphy (± heparin provocation) is a reliable modality in localizing the lesion in approximately half of OGIB cases.
Advantages and disadvantages
There are two major advantages to the use of scintigraphy. The first is that scintigraphy only requires an active bleeding rate of 0.1 to 0.4 mL/min making it the most sensitive modality in detecting an active GIB[35,36]. The second benefit is the immediate ability to test patients without any need for preparation prior to the procedure.
There are two major disadvantages to the use of scintigraphy. First, it does not offer any therapeutic capabilities, therefore in the setting of a positive scan a second procedure, i.e., endoscopy, catheter directed angiography or surgery must subsequently be performed[37]. A second major detriment to the use of scintigraphy is that it can only localize an active bleed to a general area and not a specific location. This occurs as blood moves secondary to peristaltic or anti-peristaltic actions. As a result, accuracy rates in the literature have varied ranging from 24%-91%[38-40]. Hunter et al[41] demonstrated these difficulties by evaluating the outcome of 203 patients who underwent scintigraphy for LGIB. Scintigraphy was positive in 52 patients (26%), who subsequently underwent further evaluation. A definitive bleeding site was identified in 22 patients with only 8 cases correlating to scintigraphy.
Multiphasic multidetector computed topography angiography
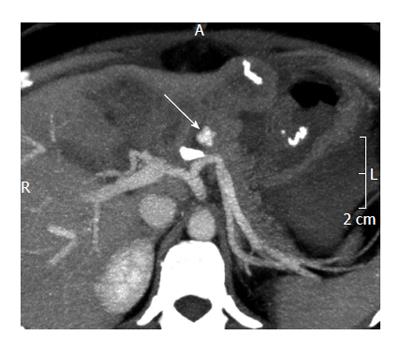
Until recently, computed topography only played a minor role in the evaluation of GIB. The introduction of multiphasic multidetector computed topography angiography (MDCTA) transformed this modality into an important tool in detecting, localizing, and characterizing active GIB. Most institutions utilize MDCTA with a 64-dector-row scanner in three phases: unenhanced, arterial, and portal-venous[42]. Comparing the arterial and portal-venous phase images to the unenhanced images is critically important in avoiding false-positive results. Active GIB often appears as extravasated contrast material in the bowel lumen or bowel wall during the arterial phase and increases throughout the portal-venous phase (> 90 HU, but typically ranging from 115-300 HU)[42-45]. Figures 4 and 5 depict the various phases of MDCTA in the setting of an active arterial bleed anterior to the splenic artery.
Figure 4 Unenhanced computed tomography showing contrast media extravasation anterior to the splenic artery[61].
Figure 5 Multiphasic multidetector computed topography angiography (arterial phase) showing extravasation anterior to the splenic artery[61].
Wu et al[46] performed a meta-analysis evaluating the accuracy of CTA in the diagnosis of acute GIB, which included a total of 9 studies with 198 patients in total. The pooled results showed a sensitivity of 89% and specificity of 85% proving MDCTA to be an accurate tool in the setting of an acute GIB. Recently published were the results of a 5-year prospective trial evaluating the utility of MDCTA in active GIB[47]. There were a total of 113 patients enrolled with clinical signs of active GIB who underwent MDCTA. The investigators found the overall sensitivity, specificity, positive and negative predictive values of MDCTA to be 86%, 100%, 100%, 61% and 89%, respectively, concluding that MDCTA is an accurate first line screening method for detection and localization of GIB.
Advantages and disadvantages
Angiography requires active blood loss of at least 0.5 mL/min[48] in order to visualize the bleeding vessel. The major advantage of MDCTA over other modalities is that it does not require any preparation and yet localization is accurate.
A major disadvantage to the use of MDCTA is the substantial radiation exposure the patient incurs. Other serious complications include cardiac arrhythmias, bowel ischemia, and a rebleeding rate in as high as 50% of patients[49]. Lastly, the use of contrast media often times precludes patients with renal failure. As always, the risk benefit ratio must be taken into account of worsening renal insufficiency vs on-going GIB. The literature varies in defining a creatinine clearance (CrCl) (most institution use a threshold value for creatinine of 1.7 and CrCl of 45 mL/min) precluding patients from MDCTA, however pre-procedural hydration and administration of n-acetylcysteine has been proven to prevent contrast induced nephropathy and worsening renal function in high-risk patients[50]. It should be noted that there are no contraindications to the use of intravenous contrast for end-stage renal patients on chronic hemodialysis.
Computed tomography enterography and magnetic resonance enterography
Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) are non-invasive alternatives used to determine the etiology of OGIB, particularly small bowel pathology[51]. Huprich et al[52] evaluated the findings of CTE in 22 outpatients with OGIB[53]. This retroactive study compared findings on CTE with capsule and traditional endoscopic, surgical, and angiographic findings. CTE findings were positive for a bleeding source in 45% of patients, with 80% of findings also positive with subsequent capsule endoscopy or clinical diagnosis. In addition, CTE correctly identified 3 lesions undetected by capsule endoscopy. A recent prospective study evaluated the use of CTE in 35 patients with OGIB, both overt (n = 20) and occult (n = 15)[54]. They found positive findings in 46.9% of CTE scans and as a result 12 patients underwent subsequent laparotomy. The surgical findings in all 12 cases were in conformity with the findings on CTE leading the authors to conclude CTE to be a useful diagnostic tool in the evaluation of both overt and occult OGIB. Since MRE is relatively new to clinical practice, there is limited data available for its use in GIB and thus may be an area of further research in the future.
Advantages and disadvantages
There are several advantages to the use of CTE and MRE, namely the ability to visualize the thickness of the bowel wall in its entirety and provide a global overview of visceral structures within the abdomen[54]. This allows for not only diagnosis but also staging of small bowel pathology.
The major disadvantage of CTE, but not MRE, is the radiation exposure to the patient[48].
Another detriment to the use of both CTE and MRE is that they do not have the necessary capabilities to perform therapeutic maneuvers. In addition, pre-existing high attenuation material within the bowel may decrease the diagnostic yield of CTE. Lastly, patients with on chronic hemodialysis should be precluded from receiving gadolinium due to risk of nephrogenic systemic fibrosis.
Provocative angiography
Provocative angiography is a salvage method used to localize the source of bleeding in patients with OGIB when all other radiologic and endoscopic approaches have been exhausted[55]. The technique involves the addition of anticoagulants, vasodilators and fibrinolytics during angiography to induce a hemorrhagic state with intent to localize the bleeding vessel.
The use of provocative angiography remains controversial due to theoretical increased risk of exacerbating the bleed. A recent prospective analysis evaluated the diagnostic yield and complication rate in 36 patients with LGIB who underwent provocative angiography[56]. Each patient was subjected to heparin followed by selective transcatheter injection of a vasodilator and plasminogen activator into the vessel of high suspicion. They found that 31% of angiograms resulted in visible extravasation and the identification of a source of LGIB in 33% of cases overall. A total of 31% underwent successful definitive treatment of LGIB with only one embolization-related complication requiring surgical resection. This led the authors to conclude provocative angiography to be a safe and effective means for eliciting the source of occult LGIB leading to a definitive therapy in up to 33% of patients.
The bottom line
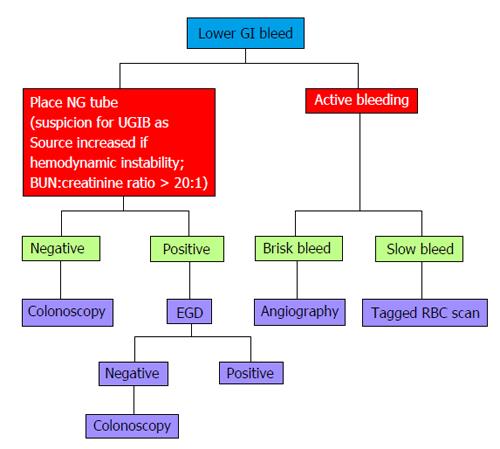
Colonoscopy remains the mainstay to diagnose the etiology of LGIB in most patients who are hemodynamically stable. In a hemodynamically unstable patient with active bleeding, scintigraphy and MDCTA are two radiologic approaches that can be utilized to identify the source. In a patient with intermittent GIB or OGIB, scintigraphy and MDCTA may prove futile. In this case, capsule endoscopy, single-balloon/double-balloon/spiral enteroscopy, and CTE are modalities used to identify and potentially treat the underlying lesion. Provocative angiography is a last resort tool when all other endoscopic and radiologic alternatives have been exhausted. There is relatively limited data on the use of MRE in OGIB, and thus may be an area requiring further research. Figure 6 provides an algorithmic approach to a patient with LGIB.
Figure 6 Algorithmic approach to lower gastrointestinal bleeding.
EGD: Esophagoduodeonscopy; GI: Gastrointestinal; NG: Nasogastric; BUN: Blood urea nitrogen; RBC: Red blood cell; UGIB: Upper gastrointestinal bleeding.
THE ROLE OF ENDOSCOPIC MARKINGS
Gastroenterologists may facilitate interventional radiologic procedures through the placement of endoscopic markings typically involving a MRI compatible metallic clip. Eriksson et al[57] recently evaluated the utility of endoscopic marking with a metallic clip in TAE for patients with UGIB. They placed a metallic clip in the fibrous edge of the ulcer adjacent to the bleeding point in 13 patients. In 10 patients, subsequent TAE was indicated due to persistent or recurrent bleeding. The artery was embolized with microcoils in close proximity to the clip. Of the 10 patients, hemostasis was achieved in 8 patients and in 6 the clip was essential in identifying the bleeding vessel. Another recent prospective study evaluated the utility of rotational angiography after endoscopic marking in patients with acute bleeding ulcers with similar results[58].
The bottom line
Endoscopic markings facilitate interventional radiologic procedure by helping to accurately localize the bleeding focus thus enhancing the possibility that the correct vessel is embolized. This can minimize the risk of recurrent bleeding after embolization and preclude further unnecessary procedures.