Copyright
©The Author(s) 2020.
World J Gastrointest Pharmacol Ther. Nov 8, 2020; 11(5): 93-109
Published online Nov 8, 2020. doi: 10.4292/wjgpt.v11.i5.93
Published online Nov 8, 2020. doi: 10.4292/wjgpt.v11.i5.93
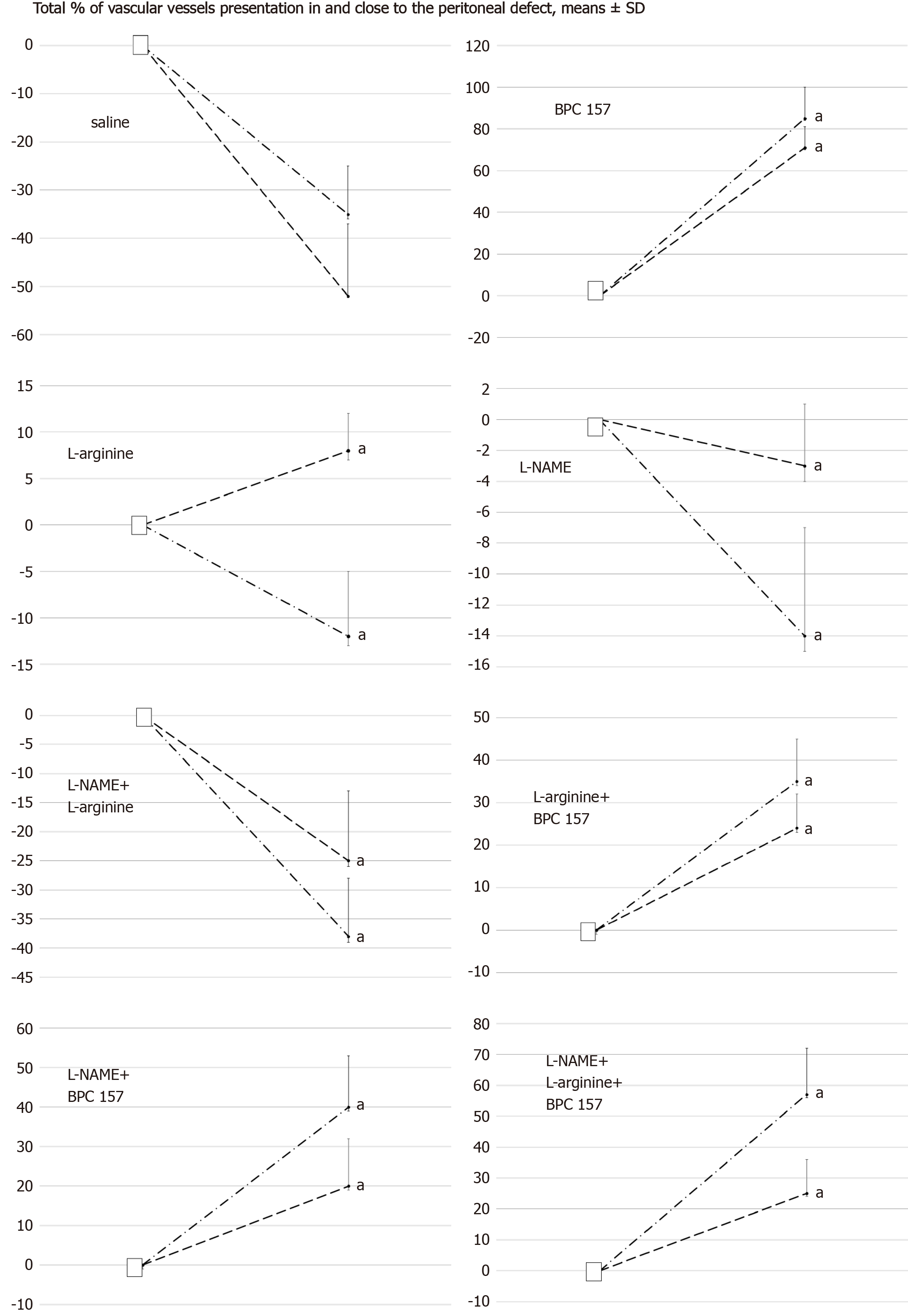
Figure 6 Total % of vascular presentation in (dash) and close to (dash dot) the defect.
White squere indicates the values immediately before therapy and full oval the values at the end of the next 10 min. Medication (/kg, 1 mL bath/rat) at abdominal cavity was BPC 157 (10 µg) (B), L-arginine (A) (100 mg), L-NAME (5 mg) alone or in combinations (L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157 (NB), L-arginine + L-NAME + BPC 157 (ANB) or saline bath equal volume (controls) (C). aP < 0.05 at least vs control; BPC 10 ng presented values 20 ± 8 (in) and 35 ± 8 (close to) with L-arginine (L-arginine + BPC 157); 25 ± 9 (in) and 45 ± 8 (close to) with L-NAME (L-NAME + BPC 157); 20 ± 7 (in) and 55 ± 15 with L-arginine and L-NAME (L-arginine + L-NAME + BPC 157), aP < 0.05 at least vs control.
- Citation: Berkopic Cesar L, Gojkovic S, Krezic I, Malekinusic D, Zizek H, Batelja Vuletic L, Petrovic A, Horvat Pavlov K, Drmic D, Kokot A, Vlainic J, Seiwerth S, Sikiric P. Bowel adhesion and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine in rats. World J Gastrointest Pharmacol Ther 2020; 11(5): 93-109
- URL: https://www.wjgnet.com/2150-5349/full/v11/i5/93.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v11.i5.93