Copyright
©The Author(s) 2020.
World J Gastrointest Pharmacol Ther. Nov 8, 2020; 11(5): 79-92
Published online Nov 8, 2020. doi: 10.4292/wjgpt.v11.i5.79
Published online Nov 8, 2020. doi: 10.4292/wjgpt.v11.i5.79
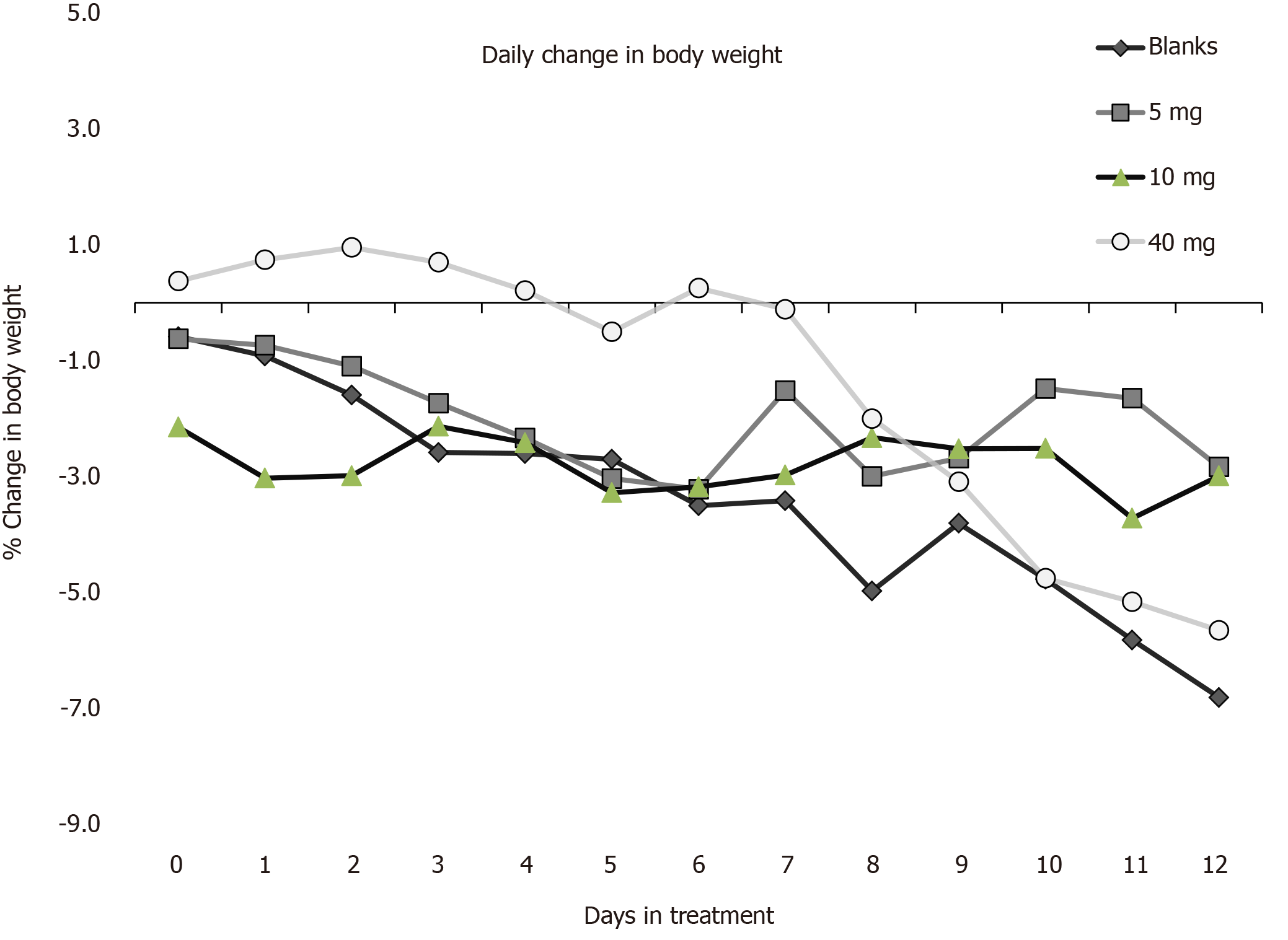
Figure 5 Therapeutic activity of transforming growth factor β loaded particles (TPX6001) in the SCID mouse adoptive CD4+ CD25- T-cell transfer model of inflammatory bowel disease.
Mice (n = 6-9 per group) with established disease were weighed (day 0) and fed transforming growth factor β1 microspheres (5, 10, or 40 mg/mouse), or blank microspheres (40 mg/mouse) in 0.2 ml water 3 times per week for 2 wk. Mice were monitored for overall disease score and weighed 3 times per week for two weeks. Mice were sacrificed 2 d after the last dose, serum taken, colons weighed and measured; and colons samples prepared for histological analysis (five randomly selected sections from each mouse). Data are expressed as % change in body weight relative to day of first treatment. 5 and 40 mg/mouse TPX6001-treated groups were significantly different (P = 0.01 on days 3-12) from animals treated with blank microspheres.
- Citation: Hammer L, Furtado S, Mathiowitz E, Auci DL. Oral encapsulated transforming growth factor β1 reduces endogenous levels: Effect on inflammatory bowel disease. World J Gastrointest Pharmacol Ther 2020; 11(5): 79-92
- URL: https://www.wjgnet.com/2150-5349/full/v11/i5/79.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v11.i5.79